Abstract
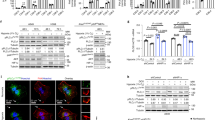
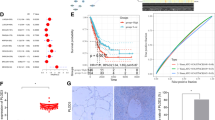
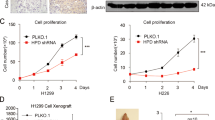
Lung cancer is the leading cause of cancer death worldwide. Recent data suggest that cyclic nucleotide phosphodiesterases (PDEs) are relevant in various cancer pathologies. Pathophysiological role of phosphodiesterase 4 (PDE4) with possible therapeutic prospects in lung cancer was investigated. We exposed 10 different lung cancer cell lines (adenocarcinoma, squamous and large cell carcinoma) to hypoxia and assessed expression and activity of PDE4 by real-time PCR, immunocytochemistry, western blotting and PDE activity assays. Expression and activity of distinct PDE4 isoforms (PDE4A and PDE4D) increased in response to hypoxia in eight of the studied cell lines. Furthermore, we analyzed various in silico predicted hypoxia-responsive elements (p-HREs) found in in PDE4A and PDE4D genes. Performing mutation analysis of the p-HRE in luciferase reporter constructs, we identified four functional HRE sites in the PDE4A gene and two functional HRE sites in the PDE4D gene that mediated hypoxic induction of the reporter. Silencing of hypoxia-inducible factor subunits (HIF1α and HIF2α) by small interfering RNA reduced hypoxic induction of PDE4A and PDE4D. Vice versa, using a PDE4 inhibitor (PDE4i) as a cyclic adenosine monophosphate (cAMP) -elevating agent, cAMP analogs or protein kinase A (PKA)-modulating drugs and an exchange protein directly activated by cAMP (EPAC) activator, we demonstrated that PDE4-cAMP-PKA/EPAC axis enhanced HIF signaling as measured by HRE reporter gene assay, HIF and HIF target genes expression ((lactate dehydrogenase A), LDHA, (pyruvate dehydrogenase kinase 1) PDK1 and (vascular endothelial growth factor A) VEGFA). Notably, inhibition of PDE4 by PDE4i or silencing of PDE4A and PDE4D reduced human lung tumor cell proliferation and colony formation. On the other hand, overexpression of PDE4A or PDE4D increased human lung cancer proliferation. Moreover, PDE4i treatment reduced hypoxia-induced VEGF secretion in human cells. In vivo, PDE4i inhibited tumor xenograft growth in nude mice by attenuating proliferation and angiogenesis. Our findings suggest that PDE4 is expressed in lung cancer, crosstalks with HIF signaling and promotes lung cancer progression. Thus, PDE4 may represent a therapeutic target for lung cancer therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ Cancer statistics 2009 CA Cancer J Clin, 2009; 59: 225–249.
Pallis AG, Serfass L, Dziadziusko R, van Meerbeeck JP, Fennell D, Lacombe D et al. Targeted therapies in the treatment of advanced/metastatic NSCLC. Eur J Cancer, 2009; 45: 2473–2487.
Rossi A, Maione P, Colantuoni G, Ferrara C, Rossi E, Guerriero C et al. Recent developments of targeted therapies in the treatment of non-small cell lung cancer. Curr Drug Discov Technol, 2009; 6: 91–102.
Yamanaka Y, Mammoto T, Kirita T, Mukai M, Mashimo T, Sugimura M et al. Epinephrine inhibits invasion of oral squamous carcinoma cells by modulating intracellular cAMP. Cancer Lett, 2002; 176: 143–148.
Timoshenko AV, Xu G, Chakrabarti S, Lala PK, Chakraborty C Role of prostaglandin E2 receptors in migration of murine and human breast cancer cells. Exp Cell Res, 2003; 289: 265–274.
McEwan DG, Brunton VG, Baillie GS, Leslie NR, Houslay MD, Frame MC Chemoresistant KM12C colon cancer cells are addicted to low cyclic AMP levels in a phosphodiesterase 4-regulated compartment via effects on phosphoinositide 3-kinase. Cancer Res, 2007; 67: 5248–5257.
Deguchi A, Thompson WJ, Weinstein IB Activation of protein kinase G is sufficient to induce apoptosis and inhibit cell migration in colon cancer cells. Cancer Res, 2004; 64: 3966–3973.
Zhang L, Murray F, Zahno A, Kanter JR, Chou D, Suda R et al. Cyclic nucleotide phosphodiesterase profiling reveals increased expression of phosphodiesterase 7B in chronic lymphocytic leukemia. Proc Natl Acad Sci USA., 2008; 105: 19532–19537.
Marko D, Romanakis K, Zankl H, Furstenberger G, Steinbauer B, Eisenbrand G Induction of apoptosis by an inhibitor of cAMP-specific PDE in malignant murine carcinoma cells overexpressing PDE activity in comparison to their nonmalignant counterparts. Cell Biochem Biophys, 1998; 28: 75–101.
Omori K, Kotera J Overview of PDEs and their regulation. Circ Res, 2007; 100: 309–327.
Conti M, Beavo J Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu Rev Biochem, 2007; 76: 481–511.
Conti M, Richter W, Mehats C, Livera G, Park JY, Jin C Cyclic AMP-specific PDE4 phosphodiesterases as critical components of cyclic AMP signaling. J Biol Chem, 2003; 278: 5493–5496.
Marko D, Pahlke G, Merz KH, Eisenbrand G Cyclic 3′,5′-nucleotide phosphodiesterases: potential targets for anticancer therapy. Chem Res Toxicol, 2000; 13: 944–948.
Mouratidis PX, Colston KW, Bartlett JB, Muller GW, Man HW, Stirling D et al. Antiproliferative effects of CC-8062 and CC-8075 in pancreatic cancer cells. Pancreas, 2009; 38: 78–84.
Narita M, Murata T, Shimizu K, Nakagawa T, Sugiyama T, Inui M et al. A role for cyclic nucleotide phosphodiesterase 4 in regulation of the growth of human malignant melanoma cells. Oncol Rep, 2007; 17: 1133–1139.
Murata K, Sudo T, Kameyama M, Fukuoka H, Muka M, Doki Y et al. Cyclic AMP specific phosphodiesterase activity and colon cancer cell motility. Clin Exp Metastasis, 2000; 18: 599–604.
Goldhoff P, Warrington NM, Limbrick DD, Hope A, Woerner BM, Jackson E et al. Targeted inhibition of cyclic AMP phosphodiesterase-4 promotes brain tumor regression. Clin Cancer Res, 2008; 14: 7717–7725.
Lerner A, Epstein PM Cyclic nucleotide phosphodiesterases as targets for treatment of haematological malignancies. Biochem J, 2006; 393(Pt 1): 21–41.
Harris AL Hypoxia–a key regulatory factor in tumour growth. Nat Rev Cancer, 2002; 2: 38–47.
Semenza GL Regulation of physiological responses to continuous and intermittent hypoxia by hypoxia-inducible factor 1. Exp Physiol, 2006; 91: 803–806.
Sowter HM, Raval RR, Moore JW, Ratcliffe PJ, Harris AL Predominant role of hypoxia-inducible transcription factor (Hif)-1alpha versus Hif-2alpha in regulation of the transcriptional response to hypoxia. Cancer Res, 2003; 63: 6130–6134.
Kolosionek E, Savai R, Ghofrani HA, Weissmann N, Guenther A, Grimminger F et al. Expression and activity of phosphodiesterase isoforms during epithelial mesenchymal transition: the role of phosphodiesterase 4. Mol Biol Cell, 2009; 20: 4751–4765.
Swinson DE, O’Byrne KJ Interactions between hypoxia and epidermal growth factor receptor in non-small-cell lung cancer. Clin Lung Cancer, 2006; 7: 250–256.
Hung JJ, Yang MH, Hsu HS, Hsu WH, Liu JS, Wu KJ Prognostic significance of hypoxia-inducible factor-1alpha, TWIST1 and Snail expression in resectable non-small cell lung cancer. Thorax, 2009; 64: 1082–1089.
Wenger RH, Stiehl DP, Camenisch G Integration of oxygen signaling at the consensus HRE. Sci STKE, 2005; 2005: re12.
Millen J, MacLean MR, Houslay MD Hypoxia-induced remodelling of PDE4 isoform expression and cAMP handling in human pulmonary artery smooth muscle cells. Eur J Cell Biol, 2006; 85: 679–691.
Nunes AR, Batuce JR, Monterio EC Functional characterization of phosphodiesterases 4 in the rat carotid body: effect of oxygen concentrations. Adv Exp Med Biol, 2009; 648: 113–119.
Chen TC, Wadsten P, Su S, Rawlinson N, Hofman FM, Hill CK et al. The type IV phosphodiesterase inhibitor rolipram induces expression of the cell cycle inhibitors p21(Cip1) and p27(Kip1), resulting in growth inhibition, increased differentiation, and subsequent apoptosis of malignant A-172 glioma cells. Cancer Biol Ther, 2002; 1: 268–276.
Ogawa R, Streiff MB, Bugayenko A, Kato GJ Inhibition of PDE4 phosphodiesterase activity induces growth suppression, apoptosis, glucocorticoid sensitivity, p53, and p21(WAF1/CIP1) proteins in human acute lymphoblastic leukemia cells. Blood, 2002; 99: 3390–3397.
Dastidar SG, Rajagopal D, Ray A Therapeutic benefit of PDE4 inhibitors in inflammatory diseases. Curr Opin Investig Drugs, 2007; 8: 364–372.
Lipworth BJ Phosphodiesterase-4 inhibitors for asthma and chronic obstructive pulmonary disease. Lancet, 2005; 365: 167–175.
Cox G, Walker RA, Andi A, Steward WP, O'Byrne KJ Prognostic significance of platelet and microvessel counts in operable non-small cell lung cancer. Lung Cancer, 2000; 29: 169–177.
Malec V, Gottschald OR, Li S, Rose F, Seeger W, Hanze J HIF-1 alpha signaling is augmented during intermittent hypoxia by induction of the Nrf2 pathway in NOX1-expressing adenocarcinoma A549 cells. Free Radic Biol Med, 2010; 48: 1626–1635.
Savai R, Schermuly RT, Voswinckel R, Renigunta A, Reichmann B, Eul B et al. HIF-1alpha attenuates tumor growth in spite of augmented vascularization in an A549 adenocarcinoma mouse model. Int J Oncol, 2005; 27: 393–400.
Acknowledgements
The authors thank Marianne Hoeck and Katharina Weidl for her excellent technical assistance. Dr Rajkumar Savai’s research was supported by the STARTUP AWARD from Justus-Liebig University Giessen, Germany and LOEWE-Center UGMLC (Universities of Giessen and Marburg Lung Center).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Pullamsetti, S., Banat, G., Schmall, A. et al. Phosphodiesterase-4 promotes proliferation and angiogenesis of lung cancer by crosstalk with HIF. Oncogene 32, 1121–1134 (2013). https://doi.org/10.1038/onc.2012.136
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2012.136
Keywords
This article is cited by
-
cAMP-PKA/EPAC signaling and cancer: the interplay in tumor microenvironment
Journal of Hematology & Oncology (2024)
-
Genome-wide interrogation of structural variation reveals novel African-specific prostate cancer oncogenic drivers
Genome Medicine (2022)
-
mGWAS identification of six novel single nucleotide polymorphism loci with strong correlation to gastric cancer
Cancer & Metabolism (2021)
-
Constructing gene regulatory networks using epigenetic data
npj Systems Biology and Applications (2021)
-
Secondary resistance to anti-EGFR therapy by transcriptional reprogramming in patient-derived colorectal cancer models
Genome Medicine (2021)