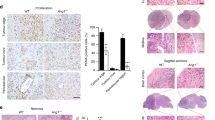
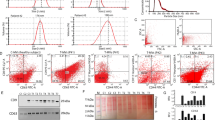
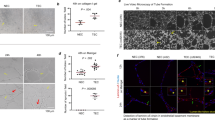
Abstract
Brain-specific angiogenesis inhibitor 1 (BAI1), an orphan G protein-coupled receptor-type seven transmembrane protein, was recently found mutated or silenced in multiple human cancers and can interfere with tumor growth when overexpressed. Yet, little is known about its regulation and the molecular mechanisms through which this novel tumor suppressor exerts its anti-cancer effects. Here, we demonstrate that the N terminus of BAI1 is cleaved extracellularly to generate a truncated receptor and a 40-kDa fragment (Vasculostatin-40) that inhibits angiogenesis. We demonstrate that this novel proteolytic processing event depends on a two-step cascade of protease activation: proprotein convertases, primarily furin, activate latent matrix metalloproteinase-14, which then directly cleaves BAI1 to release the bioactive fragment. These findings significantly augment our knowledge of BAI1 by showing a novel post-translational mechanism regulating BAI1 activity through cancer-associated proteases, have important implications for BAI1 function and regulation, and present novel opportunities for therapy of cancer and other vascular diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Kaur B, Brat DJ, Calkins CC, Van Meir EG . Brain angiogenesis inhibitor 1 is differentially expressed in normal brain and glioblastoma independently of p53 expression. Am J Pathol 2003; 162: 19–27.
Nam DH, Park K, Suh YL, Kim JH . Expression of VEGF and brain specific angiogenesis inhibitor-1 in glioblastoma: prognostic significance. Oncol Rep 2004; 11: 863–869.
Yoshida Y, Oshika Y, Fukushima Y, Tokunaga T, Hatanaka H, Kijima H et al. Expression of angiostatic factors in colorectal cancer. Int J Oncol 1999; 15: 1221–1225.
Fukushima Y, Oshika Y, Tsuchida T, Tokunaga T, Hatanaka H, Kijima H et al. Brain-specific angiogenesis inhibitor 1 expression is inversely correlated with vascularity and distant metastasis of colorectal cancer. Int J Oncol 1998; 13: 967–970.
Hatanaka H, Oshika Y, Abe Y, Yoshida Y, Hashimoto T, Handa A et al. Vascularization is decreased in pulmonary adenocarcinoma expressing brain-specific angiogenesis inhibitor 1 (BAI1). Int J Mol Med 2000; 5: 181–183.
Kudo S, Konda R, Obara W, Kudo D, Tani K, Nakamura Y et al. Inhibition of tumor growth through suppression of angiogenesis by brain-specific angiogenesis inhibitor 1 gene transfer in murine renal cell carcinoma. Oncol Rep 2007; 18: 785–791.
Duda DG, Sunamura M, Lozonschi L, Yokoyama T, Yatsuoka T, Motoi F et al. Overexpression of the p53-inducible brain-specific angiogenesis inhibitor 1 suppresses efficiently tumour angiogenesis. Br J Cancer 2002; 86: 490–496.
Yoon KC, Ahn KY, Lee JH, Chun BJ, Park SW, Seo MS et al. Lipid-mediated delivery of brain-specific angiogenesis inhibitor 1 gene reduces corneal neovascularization in an in vivo rabbit model. Gene Ther 2005; 12: 617–624.
Kan Z, Jaiswal BS, Stinson J, Janakiraman V, Bhatt D, Stern HM et al. Diverse somatic mutation patterns and pathway alterations in human cancers. Nature 2010; 466: 869–873.
Zhu D, Hunter SB, Vertino PM, Van Meir EG . Overexpression of MBD2 contributes to epigenetic silencing of BAI1 in glioblastoma. Cancer Res 2011; 71: 5859–5870.
Nishimori H, Shiratsuchi T, Urano T, Kimura Y, Kiyono K, Tatsumi K et al. A novel brain-specific p53-target gene, BAI1, containing thrombospondin type 1 repeats inhibits experimental angiogenesis. Oncogene 1997; 15: 2145–2150.
Cork SM, Van Meir EG . Emerging roles for the BAI1 protein family in the regulation of phagocytosis, synaptogenesis, neurovasculature, and tumor development. J Mol Med 2011; 89: 743–752.
Van Meir EG, Polverini PJ, Chazin VR, Huang H-JS, de Tribolet N, Cavenee WK . Release of an inhibitor of angiogenesis upon induction of wild type p53 expression in glioblastoma cells. Nat Genet 1994; 8: 171–176.
Zhang X, Lawler J . Thrombospondin-based antiangiogenic therapy. Microvasc Res 2007; 74: 90–99.
Gupta K, Gupta P, Wild R, Ramakrishnan S, Hebbel RP . Binding and displacement of vascular endothelial growth factor (VEGF) by thrombospondin: effect on human microvascular endothelial cell proliferation and angiogenesis. Angiogenesis 1999; 3: 147–158.
Dawson DW, Pearce SF, Zhong R, Silverstein RL, Frazier WA, Bouck NP . CD36 mediates the In vitro inhibitory effects of thrombospondin-1 on endothelial cells. J Cell Biol 1997; 138: 707–717.
Jimenez B, Volpert OV, Crawford SE, Febbraio M, Silverstein RL, Bouck N . Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nat Med 2000; 6: 41–48.
de Fraipont F, Nicholson AC, Feige JJ, Van Meir EG . Thrombospondins and tumor angiogenesis. Trends Mol Med 2001; 7: 401–407.
Nicholson AC, Malik SB, Logsdon Jr JM, Van Meir EG . Functional evolution of ADAMTS genes: evidence from analyses of phylogeny and gene organization. BMC Evol Biol 2005; 5: 11.
Park D, Tosello-Trampont AC, Elliott MR, Lu M, Haney LB, Ma Z et al. BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature 2007; 450: 430–434.
Kaur B, Brat DJ, Devi NS, Van Meir EG . Vasculostatin, a proteolytic fragment of brain angiogenesis inhibitor 1, is an antiangiogenic and antitumorigenic factor. Oncogene 2005; 24: 3632–3642.
Hardcastle J, Kurozumi K, Dmitrieva N, Sayers MP, Ahmad S, Waterman P et al. Enhanced antitumor efficacy of vasculostatin (Vstat120) expressing oncolytic HSV-1. Mol Ther 2010; 18: 285–294.
Kaur B, Cork SM, Sandberg EM, Devi NS, Zhang Z, Klenotic PA et al. Vasculostatin inhibits intracranial glioma growth and negatively regulates in vivo angiogenesis through a CD36-dependent mechanism. Cancer Res 2009; 69: 1212–1220.
Guedez L, Rivera AM, Salloum R, Miller ML, Diegmueller JJ, Bungay PM et al. Quantitative assessment of angiogenic responses by the directed in vivo angiogenesis assay. Am J Pathol 2003; 162: 1431–1439.
Takahashi S, Nakagawa T, Kasai K, Banno T, Duguay SJ, Van de Ven WJ et al. A second mutant allele of furin in the processing-incompetent cell line, LoVo. Evidence for involvement of the homo B domain in autocatalytic activation. J Biol Chem 1995; 270: 26565–26569.
Sato H, Kinoshita T, Takino T, Nakayama K, Seiki M . Activation of a recombinant membrane type 1-matrix metalloproteinase (MT1-MMP) by furin and its interaction with tissue inhibitor of metalloproteinases (TIMP)-2. FEBS Lett 1996; 393: 101–104.
Cao J, Rehemtulla A, Pavlaki M, Kozarekar P, Chiarelli C . Furin directly cleaves proMMP-2 in the trans-Golgi network resulting in a nonfunctioning proteinase. J Biol Chem 2005; 280: 10974–10980.
Duckert P, Brunak S, Blom N . Prediction of proprotein convertase cleavage sites. Protein Eng Des Sel 2004; 17: 107–112.
Thomas G . Furin at the cutting edge: from protein traffic to embryogenesis and disease. Nat Rev Mol Cell Biol 2002; 3: 753–766.
Fillmore HL, VanMeter TE, Broaddus WC . Membrane-type matrix metalloproteinases (MT-MMPs): expression and function during glioma invasion. J Neurooncol 2001; 53: 187–202.
Hotary KB, Yana I, Sabeh F, Li XY, Holmbeck K, Birkedal-Hansen H et al. Matrix metalloproteinases (MMPs) regulate fibrin-invasive activity via MT1-MMP-dependent and -independent processes. J Exp Med 2002; 195: 295–308.
Itoh Y, Takamura A, Ito N, Maru Y, Sato H, Suenaga N et al. Homophilic complex formation of MT1-MMP facilitates proMMP-2 activation on the cell surface and promotes tumor cell invasion. Embo J 2001; 20: 4782–4793.
Turk BE, Huang LL, Piro ET, Cantley LC . Determination of protease cleavage site motifs using mixture-based oriented peptide libraries. Nat Biotechnol 2001; 19: 661–667.
Bjarnadottir TK, Fredriksson R, Hoglund PJ, Gloriam DE, Lagerstrom MC, Schioth HB . The human and mouse repertoire of the adhesion family of G-protein-coupled receptors. Genomics 2004; 84: 23–33.
Lin HH, Chang GW, Davies JQ, Stacey M, Harris J, Gordon S . Autocatalytic cleavage of the EMR2 receptor occurs at a conserved G protein-coupled receptor proteolytic site motif. J Biol Chem 2004; 279: 31823–31832.
Krasnoperov V, Lu Y, Buryanovsky L, Neubert TA, Ichtchenko K, Petrenko AG . Post-translational proteolytic processing of the calcium-independent receptor of alpha-latrotoxin (CIRL), a natural chimera of the cell adhesion protein and the G protein-coupled receptor. Role of the G protein-coupled receptor proteolysis site (GPS) motif. J Biol Chem 2002; 277: 46518–46526.
Jin Z, Tietjen I, Bu L, Liu-Yesucevitz L, Gaur SK, Walsh CA et al. Disease-associated mutations affect GPR56 protein trafficking and cell surface expression. Hum Mol Genet 2007; 16: 1972–1985.
Hsiao CC, Cheng KF, Chen HY, Chou YH, Stacey M, Chang GW et al. Site-specific N-glycosylation regulates the GPS auto-proteolysis of CD97. FEBS Lett 2009; 583: 3285–3290.
Klenotic PA, Huang P, Palomo J, Kaur B, Van Meir EG, Vogelbaum MA et al. Histidine-rich glycoprotein modulates the anti-angiogenic effects of vasculostatin. Am J Pathol 2010; 176: 2039–2050.
Yee KO, Streit M, Hawighorst T, Detmar M, Lawler J . Expression of the type-1 repeats of thrombospondin-1 inhibits tumor growth through activation of transforming growth factor-beta. Am J Pathol 2004; 165: 541–552.
Belkin AM, Akimov SS, Zaritskaya LS, Ratnikov BI, Deryugina EI, Strongin AY . Matrix-dependent proteolysis of surface transglutaminase by membrane-type metalloproteinase regulates cancer cell adhesion and locomotion. J Biol Chem 2001; 276: 18415–18422.
Sato H, Takino T, Okada Y, Cao J, Shinagawa A, Yamamoto E et al. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature 1994; 370: 61–65.
Bassi DE, Mahloogi H, Al-Saleem L, Lopez De Cicco R, Ridge JA, Klein-Szanto AJ . Elevated furin expression in aggressive human head and neck tumors and tumor cell lines. Mol Carcinog 2001; 31: 224–232.
Mercapide J, Lopez De Cicco R, Bassi DE, Castresana JS, Thomas G, Klein-Szanto AJ . Inhibition of furin-mediated processing results in suppression of astrocytoma cell growth and invasiveness. Clin Cancer Res 2002; 8: 1740–1746.
Ishii N, Maier D, Merlo A, Tada M, Sawamura Y, Diserens AC et al. Frequent co-alterations of TP53, p16/CDKN2A, p14ARF, PTEN tumor suppressor genes in human glioma cell lines. Brain Pathol 1999; 9: 469–479.
Beck-Sickinger AG, Grouzmann E, Hoffmann E, Gaida W, Van Meir EG, Waeber B et al. A novel cyclic analog of neuropeptide Y specific for the Y2 receptor. Eur J Biochem 1992; 206: 957–964.
Acknowledgements
Paper contributions. SMC performed Vstat40 characterization, angiogenesis, cleavage site detection and furin/MMP-14 protease identification experiments, and SMC and EGVM wrote the manuscript. NSD, BK, EMS and SK performed Vstat40 and protease identification experiments. LC and JHS performed the survival analysis. Experiments were conceived of and planned by the respective authors under the direction of EGVM. We appreciate the helpful advice and assistance of all members of the Laboratory of Molecular Neuro-Oncology. SMC was in the Emory graduate program in Neuroscience and acknowledges project guidance by her thesis committee (Drs Randy Hall, Robert McKeon, Paula Vertino and Wei Zhou). We thank Dr C Dubois (Université de Sherbrooke) for the kind gift of LoVo cells. The US National Institutes of Health (R01 CA86335 to EGVM and contract 29XS193 to JHS), P30 CA138292 (to the Winship Cancer Institute) and the Emory Neurosciences Initiative (to EGVM) contributed to the funding of this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Cork, S., Kaur, B., Devi, N. et al. A proprotein convertase/MMP-14 proteolytic cascade releases a novel 40 kDa vasculostatin from tumor suppressor BAI1. Oncogene 31, 5144–5152 (2012). https://doi.org/10.1038/onc.2012.1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2012.1
Keywords
This article is cited by
-
Decylubiquinone suppresses breast cancer growth and metastasis by inhibiting angiogenesis via the ROS/p53/ BAI1 signaling pathway
Angiogenesis (2020)
-
Spatiotemporal regulation of the GPCR activity of BAI3 by C1qL4 and Stabilin-2 controls myoblast fusion
Nature Communications (2018)
-
SheddomeDB: the ectodomain shedding database for membrane-bound shed markers
BMC Bioinformatics (2017)
-
Matrix Metalloproteinase-14 Expression and Its Prognostic Value in Cervical Carcinoma
Cell Biochemistry and Biophysics (2014)