Abstract
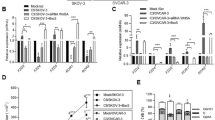
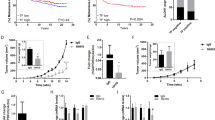
The role of the fibronectin receptor, α5β1-integrin, as an adhesion receptor and in angiogenesis is well established. However, its role in cancer cell invasion and metastasis is less clear. We describe a novel mechanism by which fibronectin regulates ovarian cancer cell signaling and promotes metastasis. Fibronectin binding to α5β1-integrin led to a direct association of α5-integrin with the receptor tyrosine kinase, c-Met, activating it in a hepatocyte growth factor/scatter factor (HGF/SF) independent manner. Subsequently, c-Met associated with Src, and activated Src and focal adhesion kinase (FAK). Inhibition of α5β1-integrin decreased the phosphorylation of c-Met, FAK and Src, both in vitro and in vivo. Independent activation of c-Met by its native ligand, HGF/SF, or overexpression of a constitutively active FAK in HeyA8 cells could overcome the effect of α5β1-integrin inhibition on tumor cell invasion, indicating that α5β1-integrin is upstream of c-Met, Src and FAK. Inhibition of α5β1-integrin on cancer cells in two xenograft models of ovarian cancer metastasis resulted in a significant decrease of tumor burden, which was independent of the effect of α5β1-integrin on angiogenesis. These data suggest that fibronectin promotes ovarian cancer invasion and metastasis through an α5β1-integrin/c-Met/FAK/Src-dependent signaling pathway, transducing signals through c-Met in an HGF/SF-independent manner.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Avraamides CJ, Garmy-Susini B, Varner JA . (2008). Integrins in angiogenesis and lymphangiogenesis. Nat Rev Cancer 8: 604–617.
Bhaskar V, Zhang D, Fox M, Seto P, Wong M, Wales PE et al. (2007). A function blocking anti-mouse integrin α5β1 antibody inhibits angiogenesis and impedes tumor growth in vivo. J Transl Med 5: 1–11.
Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF . (2003). Met, metastasis, motility and more. Nat Rev Mol Cell Biol 4: 915–925.
Brader KR, Wolf JK, Hung M-C, Yu D, Crispens MA, van Golen KL et al. (1997). Adenovirus E1A expression enhances the sensitivity of an ovarian cancer cell line to multiple cytotoxic agents through an apoptotic mechanism. Clin Cancer Res 3: 2017–2024.
Caswell PT, Spence HJ, Parsons M, White DP, Clark K, Cheng KW et al. (2007). Rab25 associates with α5β1 integrin to promote invasive migration in 3D microenvironments. Dev Cell 13: 496–510.
Chan P-C, Chen SY, Chen C-H, Chen HC . (2006). Crosstalk between hepatocyte growth factor and integrin signaling pathways. J Biomed Sci 13: 215–223.
Chan P-Y, Kanner SB, Whitney G, Aruffo A . (1994). A transmembrane-anchored chimeric focal adhesion kinase is constitutively activated and phosphorylated at tyrosine residues identical to pp125fak*. J Biol Chem 269: 20567–20574.
Chen SY, Chen H-C . (2006). Direct interaction of focal adhesion kinase (FAK) with met is required for FAK to promote hepatocyte growth factor-induced cell invasion. Mol Cell Biol 26: 5155–5167.
Francis SE, Goh KL, Hodivala-Dilke KM, Bader BL, Stark M, Davidson D et al. (2002). Central roles of α5β1 integrin and fibronectin in vascular development in mouse embryos and embryoid bodies. Arterioscler Thromb Vasc Biol 22: 927–933.
Franke FE, Von Georgi R, Zygmunt M, Münstedt K . (2003). Association between fibronectin expression and prognosis in ovarian carcinoma. Anticancer Res 23: 4261–4268.
Giancotti FG, Tarone G . (2003). Positional control of cell fate through joint integrin/receptor protein kinase signaling. Annu Rev Cell Dev Biol 19: 173–206.
Hafter R, Klaubert W, Gollwitzer R, Graeff H . (1984). Crosslinked fibrin derivatives and fibronectin in ascitic fluid from patients with ovarian cancer compared to ascitic fluid in liver cirrhosis. Thromb Haemost 35: 53–64.
Huttenlocher A, Lakonishok M, Kinder M, Wu S, Truong T, Knudsen K et al. (1998). Integrin and cadherin synergy regulates contact inhibition of migration and motile activity. 141: 515–526.
Hynes RO . (2002). Integrins: bidirectional, allosteric signaling machines. Cell 110: 673–687.
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ et al. (2009). Cancer statistics, 2009. CA Cancer J Clin 59: 225–249.
Jin E, Choi Y, Park E, Bang O, Kang S . (2007). MMP-2 functions as a negative regulator of chondrogenic cell condensation via down-regulation of the FAK -integrin β1 interaction. Devel Biol 308: 474–484.
Kenny HA, Lengyel E . (2009). MMP-2 functions as an early response protein in ovarian cancer metastasis. Cell Cycle 8: 683–688.
Kim S, Bell K, Mousa SA, Varner JA . (2000). Regulation of angiogenesis in vivo by ligation of integrin α5β1with the central cell-binding domain of fibronectin. Am J Pathol 156: 1345–1362.
Lengyel E . (2010). Ovarian cancer development and metastasis. Am J Pathol 177: 1053–1064.
Magnussen A, Kasman I, Norberg S, Baluk P, Murray R, McDonald D . (2005). Rapid access of antibodies to α5β1 integrin overexpressed on the luminal surface of tumor blood vessels. Cancer Res 65: 2712–2721.
Mitra SK, Schlaepfer D . (2006). Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol 18: 516–523.
Nakamura Y, Matsubara D, Goto A, Ota S, Sachiko O, Ishikawa S et al. (2008). Constitutive activation of c-Met is correlated with c-Met overexpression and dependent on cell-matrix adhesion in lung adenocarcinoma cell lines. Cancer Sci 99: 14–22.
Radjabi AR, Sawada K, Jagadeeswaran S, Eichbichler A, Kenny HA, Montag A et al. (2008). Thrombin induces tumor invasion through the induction and association of matrix metalloproteinase-9 and β1-integrin on the cell surface. J Biol Chem 283: 1463–1472.
Ricono JM, Huang M, Barnes LA, Lau SK, Weis S, Schlaepfer D et al. (2009). Specific cross-talk between epidermal growth factor receptor and integrin αvβ5 promotes carcinoma cell invasion and metastasis. Cancer Res 69: 1383–1391.
Rusciano D, Lorenzoni P, Burger MM . (1996). Constitutive activation of c-Met in liver metastatic B16 melanoma cells depends on both substrate adhesion and cell density and is regulated by a cytosolic tyrosine phosphatase activity. J Biol Chem 271: 20763–20769.
Saga Y, Mizukami H, Suzuki H, Urabe M, Kume A, Nakamura T et al. (2001). Expression of HGF/NK4 in ovarian cancer cells suppresses intraperitoneal dissemination and extends host survival. Gene Ther 8: 1450–1455.
Sawada K, Mitra AK, Radjabi AR, Bhaskar V, Kistner E, Tretiakova MS et al. (2008). Loss of E-cadherin promotes ovarian cancer metastasis via alpha 5-integrin, which is a therapeutic target. Cancer Res 68: 2329–2339.
Sawada K, Radjabi AR, Shinomiya N, Kistner E, Kenny HA, Salgia R et al. (2007). C-Met overexpression is a prognostic factor in ovarian cancer and an effective target for inhibition of peritoneal dissemination and invasion. Cancer Res 67: 1670–1680.
Taverna D, Hynes RO . (2001). Reduced blood vessel formation and tumor growth in α5-integrin-negative teratocarcinomas and embryoid bodies. Cancer Res 61: 5255–5261.
Trusolino L, Bertotti A, Comoglio PM . (2001). A signaling adapter function for α6β4 integrin in the control of HGF-dependent invasive growth. Cell 107: 643–654.
Vuori K, Ruoslahti E . (1994). Association of insulin receptor substrate-1 with integrins. Science 266: 1576–1578.
Wang R, Kobayashi R, Bishop M . (1996). Cellular adherence elicits ligand-independent activation of the Met cell -surface receptor. Proc Natl Acad Sci USA 93: 8425–8430.
Wingerter P, Kazman I, Norberg S, Magnussen A, Zanivan S, Rissone A et al. (2005). Uniform overexpression and rapid accessibility of α5β1 integrin on blood vessels in tumors. Am J Pathol 167: 193–211.
Wong AST, Roskelley CD, Pelech SL, Miller D, Leung PCK, Auersperg N . (2004). Progressive changes in Met-dependent signaling in a human ovarian surface epithelial model of malignant transformation. Exp Cell Res 299: 248–256.
Yang JT, Rayburn H, Hynes RO . (1993). Embryonic mesodermal defects in α5 integrin-deficient mice. Development 119: 1093–1105.
Yokoyama Y, Sedgewick G, Ramakrishnan S . (2007). Endostatin binding to ovarian cancer cells inhibits peritoneal attachment and dissemination. Cancer Res 67: 10813–10822.
Yu D, Wolf JK, Scanlon M, Price JE, Hung M-C . (1992). Enhanced c-erb B-2/neu expression in human ovarian cancer cells correlates with more severe malignancy that can be suppressed by E1A. Cancer Res 53: 891–898.
Zillhardt M, Christensen J, Lengyel E . (2010). An orally available small molecule inhibitor of c-Met, PF-2341066, reduces tumor burden in a pre-clinical model of ovarian cancer metastasis. Neoplasia 12: 1–10.
Acknowledgements
We thank Dr Alan Horwitz, Department of Cell Biology, University of Virginia School of Medicine for providing us with the FAK constructs. We thank Gail Isenberg for critically reviewing the manuscript. We appreciate the inputs of Dr Vinay Bhaskar and Dr Vanitha Ramakrishnan during the initial part of the project. Funding: Ernst Lengyel holds a Clinical Scientist Award in Translational Research from the Burroughs Wellcome Fund and is supported by grants from the Ovarian Cancer Research Fund (Liz Tilberis Scholars Program), and the NCI (RO1 CA111882).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Facet Biotech Corp. provided funding and antibodies for the animal experiments (Figure 1).
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Mitra, A., Sawada, K., Tiwari, P. et al. Ligand-independent activation of c-Met by fibronectin and α5β1-integrin regulates ovarian cancer invasion and metastasis. Oncogene 30, 1566–1576 (2011). https://doi.org/10.1038/onc.2010.532
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2010.532
Keywords
This article is cited by
-
The interaction of β-arrestin1 with talin1 driven by endothelin A receptor as a feature of α5β1 integrin activation in high-grade serous ovarian cancer
Cell Death & Disease (2023)
-
Structural analysis of peptide binding to integrins for cancer detection and treatment
Biophysical Reviews (2023)
-
HOXA13 promotes gastric cancer progression partially via the FN1-mediated FAK/Src axis
Experimental Hematology & Oncology (2022)
-
L1CAM is required for early dissemination of fallopian tube carcinoma precursors to the ovary
Communications Biology (2022)
-
Wnt5A modulates integrin expression in a receptor-dependent manner in ovarian cancer cells
Scientific Reports (2021)