Abstract
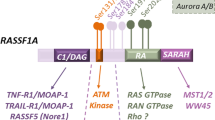
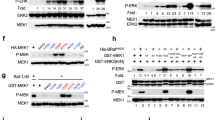
Ras association domain family protein 1A (RASSF1A) is one of the more heavily methylated genes in human cancers. In addition to promoter-specific methylation, RASSF1A polymorphisms have been identified in cancer patients. RASSF1A is a tumor suppressor protein involved in death receptor-dependent apoptosis and it is localized to microtubules. Currently, the biological importance of RASSF1A microtubule localization and the functional consequences of RASSF1A polymorphisms is not understood. In this study, we have investigated both RASSF1A microtubule association and polymorphisms. Loss of RASSF1A microtubule association resulted in the nuclear appearance of RASSF1A and the loss of association with α-, γ- and β-tubulin. Moreover, the loss of microtubule localization of RASSF1A resulted in enhanced tumor-promoting potential, as determined by a xenograft transplantation model in nude mice. It is surprising that, several RASSF1A polymorphisms also lost the ability to associate with α-, γ- and β-tubulin and lost the ability to prevent tumor formation in a xenograft nude mouse model when compared with wild-type RASSF1A. Our results demonstrate a role for RASSF1A microtubule localization in eliciting its tumor suppressor function. In addition, some RASSF1A polymorphisms lack the tumor suppressor function of RASSF1A and, if present in patients, may be tumorigenic.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Accession codes
Abbreviations
- RASSF:
-
Ras association domain family
- TNFα:
-
tumor necrosis factor α
- TNF-R1:
-
TNFα receptor R1
- MOAP-1:
-
modulator of apoptosis 1
- TRAIL:
-
TNFα apoptosis-inducing ligand
- TRAIL-R1:
-
TRAIL receptor 1
- PARP:
-
poly (ADP-ribose) polymerase
- NFκB:
-
nuclear factor κB
- APC1:
-
adenomatous polyposis coli
- GSK-3β:
-
glycogen synthase kinase 3β
- GFP:
-
green fluorescent protein
- GST:
-
glutathione S-transferase
- HA:
-
Hematagluttin
- WT:
-
wild type
- SDS–PAGE:
-
sodium dodecyl sulphate gel electrophoresis
References
Agathanggelou A, Honorio S, Macartney DP, Martinez A, Dallol A, Rader J et al. (2001). Methylation associated inactivation of RASSF1A from region 3p21.3 in lung, breast and ovarian tumours. Oncogene 20: 1509–1518.
Adachi M, Zhang YB, Imai K . (2003). Mutation of BAD within the BH3 domain impairs its phosphorylation-mediated regulation. FEBS Lett 551: 147–152.
Allen NP, Donninger H, Vos MD, Eckfeld K, Hesson L, Gordon L et al. (2007). RASSF6 is a novel member of the RASSF family of tumor suppressors. Oncogene 26: 6203–6211.
Avruch J, Xavier R, Bardeesy N, Zhang XF, Praskova M, Zhou D et al. (2009). Rassf family of tumor suppressor polypeptides. J Biol Chem 284: 11001–11005.
Baksh S, Tommasi S, Fenton S, Yu VC, Martins LM, Pfeifer GP et al. (2005). The tumor suppressor RASSF1A and MAP-1 link death receptor signaling to Bax conformational change and cell death. Mol Cell 18: 637–650.
Bhat KM, Setaluri V . (2007). Microtubule-associated proteins as targets in cancer chemotherapy. Clin Cancer Res 13: 2849–2854.
Brameier M, Krings A, MacCallum RM . (2007). NucPred--predicting nuclear localization of proteins. Bioinformatics 23: 1159–1160.
Cuschieri L, Nguyen T, Vogel J . (2007). Control at the cell center: the role of spindle poles in cytoskeletal organization and cell cycle regulation. Cell Cycle 6: 2788–2794.
Dallol A, Agathanggelou A, Fenton SL, Ahmed-Choudhury J, Hesson L, Vos MD et al. (2004). RASSF1A interacts with microtubule-associated proteins and modulates microtubule dynamics. Cancer Res 64: 4112–4116.
Dallol A, Cooper WN, Al-Mulla F, Agathanggelou A, Maher ER, Latif F . (2007). Depletion of the Ras association domain family 1, isoform A-associated novel microtubule-associated protein, C19ORF5/MAP1S, causes mitotic abnormalities. Cancer Res 67: 492–500.
Dammann R, Schagdarsurengin U, Seidel C, Strunnikova M, Rastetter M, Baier K et al. (2005). The tumor suppressor RASSF1A in human carcinogenesis: an update. Histol Histopathol 20: 645–663.
Donninger H, Vos MD, Clark GJ . (2007). The RASSF1A tumor suppressor. J Cell Sci 120: 3163–3172.
Fajas L, Paul C, Vie A, Estrach S, Medema R, Blanchard JM et al. (2001). Cyclin A is a mediator of p120E4F-dependent cell cycle arrest in G1. Mol Cell Biol 21: 2956–2966.
Fajas L, Paul C, Zugasti O, Le Cam L, Polanowska J, Fabbrizio E et al. (2000). pRB binds to and modulates the transrepressing activity of the E1A-regulated transcription factor p120E4F. Proc Natl Acad Sci USA 97: 7738–7743.
Fenton SL, Dallol A, Agathanggelou A, Hesson L, Ahmed-Choudhury J, Baksh S et al. (2004). Identification of the E1A-regulated transcription factor p120 E4F as an interacting partner of the RASSF1A candidate tumor suppressor gene. Cancer Res 64: 102–107.
Fernandes ER, Rooney RJ . (1997). The adenovirus E1A-regulated transcription factor E4F is generated from the human homolog of nuclear factor phiAP3. Mol Cell Biol 17: 1890–1903.
Fisk HA, Mattison CP, Winey M . (2002). Centrosomes and tumour suppressors. Curr Opin Cell Biol 14: 700–705.
Foley CJ, Freedman H, Choo SL, Onyskiw C, Fu NY, Yu VC et al. (2008). Dynamics of RASSF1A/MOAP-1 association with death receptors. Mol Cell Biol 28: 4520–4535.
Gao B, Xie XJ, Huang C, Shames DS, Chen TT, Lewis CM et al. (2008). RASSF1A polymorphism A133S is associated with early onset breast cancer in BRCA1/2 mutation carriers. Cancer Res 68: 22–25.
Ghazaleh HA, Chow RS, Choo SL, Pham D, Olesen JD, Wong RX et al. (2010). 14–3–3 mediated regulation of the tumor suppressor protein, RASSF1A. Apoptosis 15: 117–127.
Hamilton G, Yee KS, Scrace S, O'Neill E . (2009). ATM regulates a RASSF1A-dependent DNA damage response. Curr Biol 19: 2020–2025.
la Cour T, Kiemer L, Molgaard A, Gupta R, Skriver K, Brunak S . (2004). Analysis and prediction of leucine-rich nuclear export signals. Protein Eng Des Sel 17: 527–536.
Liu L, Tommasi S, Lee DH, Dammann R, Pfeifer GP . (2003). Control of microtubule stability by the RASSF1A tumor suppressor. Oncogene 22: 8125–8136.
Liu L, Vo A, Liu G, McKeehan WL . (2005a). Putative tumor suppressor RASSF1 interactive protein and cell death inducer C19ORF5 is a DNA binding protein. Biochem Biophys Res Commun 332: 670–676.
Liu L, Vo A, McKeehan WL . (2005b). Specificity of the methylation-suppressed A isoform of candidate tumor suppressor RASSF1 for microtubule hyperstabilization is determined by cell death inducer C19ORF5. Cancer Res 65: 1830–1838.
Lowe SW, Cepero E, Evan G . (2004). Intrinsic tumour suppression. Nature 432: 307–315.
Matallanas D, Romano D, Yee K, Meissl K, Kucerova L, Piazzolla D et al. (2007). RASSF1A elicits apoptosis through an MST2 pathway directing proapoptotic transcription by the p73 tumor suppressor protein. Mol Cell 27: 962–975.
Moshnikova A, Frye J, Shay JW, Minna JD, Khokhlatchev AV . (2006). The growth and tumor suppressor NORE1A is a cytoskeletal protein that suppresses growth by inhibition of the ERK pathway. J Biol Chem 281: 8143–8152.
Moshnikova A, Kuznetsov S, Khokhlatchev AV . (2008). Interaction of the growth and tumour suppressor NORE1A with microtubules is not required for its growth-suppressive function. BMC Res Notes 1: 13.
Parvin JD . (2009). The BRCA1-dependent ubiquitin ligase, gamma-tubulin, and centrosomes. Environ Mol Mutagen 50: 649–653.
Pihan G, Doxsey SJ . (2003). Mutations and aneuploidy: co-conspirators in cancer? Cancer Cell 4: 89–94.
Praskova M, Khoklatchev A, Ortiz-Vega S, Avruch J . (2004). Regulation of the MST1 kinase by autophosphorylation, by the growth inhibitory proteins, RASSF1 and NORE1, and by Ras. Biochem J 381: 453–462.
Raynaud-Messina B, Merdes A . (2007). Gamma-tubulin complexes and microtubule organization. Curr Opin Cell Biol 19: 24–30.
Richter AM, Pfeifer GP, Dammann RH . (2009). The RASSF proteins in cancer; from epigenetic silencing to functional characterization. Biochim Biophys Acta 1796: 114–128.
Rizos H, Diefenbach E, Badhwar P, Woodruff S, Becker TM, Rooney RJ et al. (2003). Association of p14ARF with the p120E4F transcriptional repressor enhances cell cycle inhibition. J Biol Chem 278: 4981–4989.
Rong R, Jin W, Zhang J, Sheikh MS, Huang Y . (2004). Tumor suppressor RASSF1A is a microtubule-binding protein that stabilizes microtubules and induces G2/M arrest. Oncogene 23: 8216–8230.
Senda T, Shimomura A, Iizuka-Kogo A . (2005). Adenomatous polyposis coli (Apc) tumor suppressor gene as a multifunctional gene. Anat Sci Int 80: 121–131.
Sherwood V, Manbodh R, Sheppard C, Chalmers AD . (2008). RASSF7 is a member of a new family of RAS association domain-containing proteins and is required for completing mitosis. Mol Biol Cell 19: 1772–1782.
Song MS, Chang JS, Song SJ, Yang TH, Lee H, Lim DS . (2005). The centrosomal protein RAS association domain family protein 1A (RASSF1A)-binding protein 1 regulates mitotic progression by recruiting RASSF1A to spindle poles. J Biol Chem 280: 3920–3927.
van der Weyden L, Adams DJ . (2007). The Ras-association domain family (RASSF) members and their role in human tumourigenesis. Biochim Biophys Acta 1776: 58–85.
van der Weyden L, Arends MJ, Dovey OM, Harrison HL, Lefebvre G, Conte N et al. (2008). Loss of Rassf1a cooperates with Apc(Min) to accelerate intestinal tumourigenesis. Oncogene 27: 4503–4508.
van der Weyden L, Tachibana KK, Gonzalez MA, Adams DJ, Ng BL, Petty R et al. (2005). The RASSF1A isoform of RASSF1 promotes microtubule stability and suppresses tumorigenesis. Mol Cell Biol 25: 8356–8367.
Vos MD, Ellis CA, Elam C, Ulku AS, Taylor BJ, Clark GJ . (2003a). RASSF2 is a novel K-Ras-specific effector and potential tumor suppressor. J Biol Chem 278: 28045–28051.
Vos MD, Martinez A, Elam C, Dallol A, Taylor BJ, Latif F et al. (2004). A role for the RASSF1A tumor suppressor in the regulation of tubulin polymerization and genomic stability. Cancer Res 64: 4244–4250.
Vos MD, Martinez A, Ellis CA, Vallecorsa T, Clark GJ . (2003b). The pro-apoptotic Ras effector Nore1 may serve as a Ras-regulated tumor suppressor in the lung. J Biol Chem 278: 21938–21943.
Wade RH . (2009). On and around microtubules: an overview. Mol Biotechnol 43: 177–191.
Acknowledgements
We thank Dr Haya Abu Ghazaleh for her helpful discussions, Adrienne DeCorby-Baksh for her excellent technical assistance with our confocal imaging, Dr Victor Yu and Naiyang Fu for the MOAP-1 expression construct. This work was supported by grants from CIHR (MOP-79494) (MEK), the Women and Children's Health Research Institute (WCO14) (MEK), the Stollery Children's Hospital Foundation Donation Grant (PD946) (SB and MEK), Alberta Heritage Foundation for Medical Research (G220170170) (SB), and Canadian Foundation for Innovation/Alberta Small Equipment Grants Program (#13118).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
El-Kalla, M., Onyskiw, C. & Baksh, S. Functional importance of RASSF1A microtubule localization and polymorphisms. Oncogene 29, 5729–5740 (2010). https://doi.org/10.1038/onc.2010.316
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2010.316
Keywords
This article is cited by
-
A role for RASSF1A in tunneling nanotube formation between cells through GEFH1/Rab11 pathway control
Cell Communication and Signaling (2018)
-
Dual-functionality of RASSF1A overexpression in A375 cells is mediated by activation of IL-6/STAT3 regulatory loop
Molecular Biology Reports (2018)
-
Epigenetic silencing of RASSF1A deregulates cytoskeleton and promotes malignant behavior of adrenocortical carcinoma
Molecular Cancer (2013)