Abstract
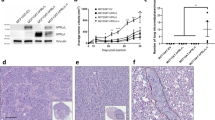
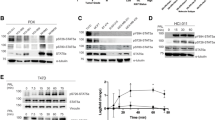
The prolactin receptor (PRLR), its associated Janus kinase 2 (Jak2) and the signal transducer and activator of transcription 5 (Stat5) are essential for normal mammary gland development. Owing to the upregulation of the PRLR and the local synthesis of its ligand in neoplastic cells, it has been proposed that PRL can act as a local growth factor in human breast cancers. This notion is supported by experimental evidence in transgenic mice, which showed that the mammary-specific expression of PRL contributes to carcinogenesis in vivo. To assess the importance of Jak2/Stat5 signaling during mammary cancer initiation and progression, we generated a PRL-induced mammary cancer model that allows the functional ablation of the Jak2 gene in the mammary epithelium before and after neoplastic transformation. Collectively, the results of this study show that the functional ablation of Jak2 protects against the onset of PRL-induced mammary tumorigenesis, suggesting that targeting this kinase is a relevant strategy for mammary cancer prevention. Surprisingly, Jak2 deficiency did not affect the growth and survival of PRL-induced mammary cancer cells in culture and in vivo. Consequently, Jak2 cannot be a sole therapeutic target to treat the established disease. PRL-induced mammary cancers exhibited an upregulation of ErbB2 and other ErbB receptor tyrosine kinases that may supersede the functionality of PRLR signaling through Jak2.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Aoui-Jamali MA, Song DJ, Benlimame N, Yen L, Deng X, Hernandez-Perez M et al. (2003). Regulation of multiple tumor microenvironment markers by overexpression of single or paired combinations of ErbB receptors. Cancer Res 63: 3764–3774.
Arendt LM, Rose-Hellekant TA, Sandgren EP, Schuler LA . (2006). Prolactin potentiates transforming growth factor alpha induction of mammary neoplasia in transgenic mice. Am J Pathol 168: 1365–1374.
Arendt LM, Schuler LA . (2008). Prolactin drives estrogen receptor-alpha-dependent ductal expansion and synergizes with transforming growth factor-alpha to induce mammary tumors in males. Am J Pathol 172: 194–202.
Boulanger CA, Wagner KU, Smith GH . (2005). Parity-induced mouse mammary epithelial cells are pluripotent, self-renewing and sensitive to TGF-beta1 expression. Oncogene 24: 552–560.
Brockman JL, Schroeder MD, Schuler LA . (2002). PRL activates the cyclin D1 promoter via the Jak2/Stat pathway. Mol Endocrinol 16: 774–784.
Brockman JL, Schuler LA . (2005). Prolactin signals via Stat5 and Oct-1 to the proximal cyclin D1 promoter. Mol Cell Endocrinol 239: 45–53.
Cardiff RD . (1998). Are the TDLU of the human the same as the LA of mice? J Mammary Gland Biol Neoplasia 3: 3–5.
Clevenger CV, Chang WP, Ngo W, Pasha TL, Montone KT, Tomaszewski JE . (1995). Expression of prolactin and prolactin receptor in human breast carcinoma. Evidence for an autocrine/paracrine loop. Am J Pathol 146: 695–705.
Cotarla I, Ren S, Zhang Y, Gehan E, Singh B, Furth PA . (2004). Stat5a is tyrosine phosphorylated and nuclear localized in a high proportion of human breast cancers. Int J Cancer 108: 665–671.
Cui Y, Riedlinger G, Miyoshi K, Tang W, Li C, Deng CX et al. (2004). Inactivation of Stat5 in mouse mammary epithelium during pregnancy reveals distinct functions in cell proliferation, survival, and differentiation. Mol Cell Biol 24: 8037–8047.
Diehl JA, Cheng M, Roussel MF, Sherr CJ . (1998). Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev 12: 3499–3511.
Dominguez-Caceres MA, Garcia-Martinez JM, Calcabrini A, Gonzalez L, Porque PG, Leon J et al. (2004). Prolactin induces c-Myc expression and cell survival through activation of Src/Akt pathway in lymphoid cells. Oncogene 23: 7378–7390.
Garcia-Martinez JM, Calcabrini A, Gonzalez L, Martin-Forero E, gullo-Ortuno MT, Simon V et al. (2010). A non-catalytic function of the Src family tyrosine kinases controls prolactin-induced Jak2 signaling. Cell Signal 22: 415–426.
Ginsburg E, Vonderhaar BK . (1995). Prolactin synthesis and secretion by human breast cancer cells. Cancer Res 55: 2591–2595.
Hankinson SE, Willett WC, Michaud DS, Manson JE, Colditz GA, Longcope C et al. (1999). Plasma prolactin levels and subsequent risk of breast cancer in postmenopausal women. J Natl Cancer Inst 91: 629–634.
Henry MD, Triplett AA, Oh KB, Smith GH, Wagner KU . (2004). Parity-induced mammary epithelial cells facilitate tumorigenesis in MMTV-neu transgenic mice. Oncogene 23: 6980–6985.
Holbro T, Beerli RR, Maurer F, Koziczak M, Barbas CF, Hynes NE . (2003). The ErbB2/ErbB3 heterodimer functions as an oncogenic unit: ErbB2 requires ErbB3 to drive breast tumor cell proliferation. Proc Natl Acad Sci USA 100: 8933–8938.
Horseman ND, Zhao W, Montecino-Rodriguez E, Tanaka M, Nakashima K, Engle SJ et al. (1997). Defective mammopoiesis, but normal hematopoiesis, in mice with a targeted disruption of the prolactin gene. EMBO J 16: 6926–6935.
Humphreys RC, Hennighausen L . (1999). Signal transducer and activator of transcription 5a influences mammary epithelial cell survival and tumorigenesis. Cell Growth Differ 10: 685–694.
Iavnilovitch E, Cardiff RD, Groner B, Barash I . (2004). Deregulation of Stat5 expression and activation causes mammary tumors in transgenic mice. Int J Cancer 112: 607–619.
Iavnilovitch E, Groner B, Barash I . (2002). Overexpression and forced activation of stat5 in mammary gland of transgenic mice promotes cellular proliferation, enhances differentiation, and delays postlactational apoptosis. Mol Cancer Res 1: 32–47.
Kim A, Liu B, Ordonez-Ercan D, Alvarez K, Jones L, McKimmey C et al. (2005). Functional interaction between mouse erbB3 and wild-type rat c-neu in transgenic mouse mammary tumor cells. Breast Cancer Res 7: R708–R718.
Krempler A, Qi Y, Triplett AA, Zhu J, Rui H, Wagner KU . (2004). Generation of a conditional knockout allele for the Janus kinase 2 (Jak2) gene in mice. Genesis 40: 52–57.
Lim E, Vaillant F, Wu D, Forrest NC, Pal B, Hart AH et al. (2009). Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nat Med 15: 907–913.
Matulka LA, Triplett AA, Wagner KU . (2007). Parity-induced mammary epithelial cells are multipotent and express cell surface markers associated with stem cells. Dev Biol 303: 29–44.
Matulka LA, Wagner KU . (2005). Models of Breast Cancer. Drug Discov Today Dis Models 2: 1–6.
Medina D, Kittrell FS . (2000). Establishment of mouse mammary cell lines. In: Ip MM, Ash BB (eds). Methods in Mammary Gland Biology and Breast Cancer, chapter 13. Kluwer Academic/Plenum Publishers: New York. pp 137–145.
Miermont AM, Parrish AR, Furth PA . (2010). Role of ERα in the differential response of Stat5a loss in susceptibility to mammary preneoplasia and DMBA-induced carcinogenesis. Carcinogenesis 31: 1124–1131.
Nevalainen MT, Xie J, Torhorst J, Bubendorf L, Haas P, Kononen J et al. (2004). Signal transducer and activator of transcription-5 activation and breast cancer prognosis. J Clin Oncol 22: 2053–2060.
Oakes SR, Robertson FG, Kench JG, Gardiner-Garden M, Wand MP, Green JE et al. (2007). Loss of mammary epithelial prolactin receptor delays tumor formation by reducing cell proliferation in low-grade preinvasive lesions. Oncogene 26: 543–553.
Ormandy CJ, Camus A, Barra J, Damotte D, Lucas B, Buteau H et al. (1997). Null mutation of the prolactin receptor gene produces multiple reproductive defects in the mouse. Genes Dev 11: 167–178.
Pardanani A . (2007). JAK2 inhibitor therapy in myeloproliferative disorders: rationale, preclinical studies and ongoing clinical trials. Leukemia 22: 23–30.
Ren S, Cai HR, Li M, Furth PA . (2002). Loss of Stat5a delays mammary cancer progression in a mouse model. Oncogene 21: 4335–4339.
Rose-Hellekant TA, Arendt LM, Schroeder MD, Gilchrist K, Sandgren EP, Schuler LA . (2003). Prolactin induces ERalpha-positive and ERalpha-negative mammary cancer in transgenic mice. Oncogene 22: 4664–4674.
Sakamoto K, Creamer BA, Triplett AA, Wagner KU . (2007). The Janus kinase 2 is required for expression and nuclear accumulation of cyclin D1 in proliferating mammary epithelial cells. Mol Endocrinol 21: 1877–1892.
Sakamoto K, Lin WC, Triplett AA, Wagner KU . (2009). Targeting janus kinase 2 in Her2/neu-expressing mammary cancer: Implications for cancer prevention and therapy. Cancer Res 69: 6642–6650.
Shillingford JM, Miyoshi K, Robinson GW, Grimm SL, Rosen JM, Neubauer H et al. (2002). Jak2 is an essential tyrosine kinase involved in pregnancy-mediated development of mammary secretory epithelium. Mol Endocrinol 16: 563–570.
Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE et al. (1989). Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 244: 707–712.
Tang JZ, Zuo ZH, Kong XJ, Steiner M, Yin Z, Perry JK et al. (2010). Signal transducer and activator of transcription (STAT)-5A and STAT5B differentially regulate human mammary carcinoma cell behavior. Endocrinology 151: 43–55.
Teglund S, McKay C, Schuetz E, van Deursen JM, Stravopodis D, Wang D et al. (1998). Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell 93: 841–850.
Tornell J, Rymo L, Isaksson OG . (1991). Induction of mammary adenocarcinomas in metallothionein promoter-human growth hormone transgenic mice. Int J Cancer 49: 114–117.
Tran TH, Utama FE, Lin J, Yang N, Sjolund AB, Ryder A et al. (2010). Prolactin inhibits BCL6 expression in breast cancer through a Stat5a-dependent mechanism. Cancer Res 70: 1711–1721.
Triplett AA, Montagna C, Wagner KU . (2008). A mammary-specific, long-range deletion on mouse chromosome 11 accelerates Brca1-associated mammary tumorigenesis. Neoplasia 10: 1325–1334.
Tworoger SS, Hankinson SE . (2006). Prolactin and breast cancer risk. Cancer Lett 243: 160–169.
Wagner KU, Boulanger CA, Henry MD, Sgagias M, Hennighausen L, Smith GH . (2002). An adjunct mammary epithelial cell population in parous females: its role in functional adaptation and tissue renewal. Development 129: 1377–1386.
Wagner KU, Krempler A, Triplett AA, Qi Y, George NM, Zhu J et al. (2004). Impaired alveologenesis and maintenance of secretory mammary epithelial cells in Jak2 conditional knockout mice. Mol Cell Biol 24: 5510–5520.
Wagner KU, McAllister K, Ward T, Davis B, Wiseman R, Hennighausen L . (2001). Spatial and temporal expression of the Cre gene under the control of the MMTV-LTR in different lines of transgenic mice. Transgenic Res 10: 545–553.
Wagner KU, Rui H . (2008). Jak2/Stat5 signaling in mammogenesis, breast cancer initiation and progression. J Mammary Gland Biol Neoplasia 13: 93–103.
Wagner KU, Wall RJ, St-Onge L, Gruss P, Wynshaw-Boris A, Garrett L et al. (1997a). Cre-mediated gene deletion in the mammary gland. Nucleic Acids Res 25: 4323–4330.
Wagner KU, Young III WS, Liu X, Ginns EI, Li M, Furth PA et al. (1997b). Oxytocin and milk removal are required for post-partum mammary-gland development. Genes Funct 1: 233–244.
Wellings SR, Jensen HM, Marcum RG . (1975). An atlas of subgross pathology of the human breast with special reference to possible precancerous lesions. J Natl Cancer Inst 55: 231–273.
Wennbo H, Gebre-Medhin M, Gritli-Linde A, Ohlsson C, Isaksson OG, Tornell J . (1997). Activation of the prolactin receptor but not the growth hormone receptor is important for induction of mammary tumors in transgenic mice. J Clin Invest 100: 2744–2751.
Acknowledgements
This work was supported by the Public Health Service Grant CA117930 (to K-UW) as well as Grant CA78312 (to LAS) from the National Cancer Institute. Additional financial support provided to K-UW by the Nebraska Cancer and Smoking Disease Research Program (NE DHHS LB506 2009-45) was imperative to finance the maintenance of the Jak2 conditional knockout model. KS received a postdoctoral fellowship from the Susan G Komen Breast Cancer Foundation (PDF0600835).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Sakamoto, K., Triplett, A., Schuler, L. et al. Janus kinase 2 is required for the initiation but not maintenance of prolactin-induced mammary cancer. Oncogene 29, 5359–5369 (2010). https://doi.org/10.1038/onc.2010.274
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2010.274
Keywords
This article is cited by
-
Identification of selective inhibitors for Janus kinase 1: an integrated drug repurposing strategy for breast cancer
Chemical Papers (2024)
-
Dual recombinase action in the normal and neoplastic mammary gland epithelium
Scientific Reports (2021)
-
Elevated collagen-I augments tumor progressive signals, intravasation and metastasis of prolactin-induced estrogen receptor alpha positive mammary tumor cells
Breast Cancer Research (2017)
-
STAT3 activation is required for the antiapoptotic effects of prolactin in cervical cancer cells
Cancer Cell International (2015)
-
The transcriptional responsiveness of LKB1 to STAT-mediated signaling is differentially modulated by prolactin in human breast cancer cells
BMC Cancer (2014)