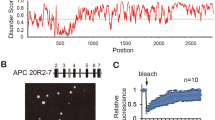
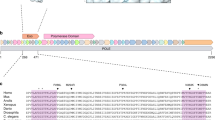
Abstract
The adenomatous polyposis coli (APC) protein is a negative regulator of the mitogenic transcription factor β-catenin by stimulating its proteasomal degradation. This involves several APC domains, including the binding sites for axin/conductin, the recently described β-Catenin Inhibitory Domain (CID) and the third 20 amino acid repeat (20R3) that is a β-catenin-binding site. The four 15 amino acid repeats (15R) and the 20R1 are also β-catenin-binding sites, but their role in β-catenin degradation has remained unclear. We show here that binding of β-catenin to the 15R of APC is necessary and sufficient to target β-catenin for degradation whereas binding to the 20R1 is neither necessary nor sufficient. The first 15R displays the highest affinity for β-catenin in the 15R-20R1 module. Biallelic mutations of the APC gene lead tocolon cancer in familial adenomatous polyposis coli (FAP) and result in the synthesis of truncated products lacking domains involved in β-catenin degradation but still having a minimal length. The analysis of the distribution of truncating mutations along the APC sequence in colorectal tumours from FAP patients revealed that the first 15R is one target of the positive selection of mutations that lead to tumour development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Akiyama T, Kawasaki Y . (2006). Wnt signalling and the actin cytoskeleton. Oncogene 25: 7538–7544.
Albuquerque C, Breukel C, van der Luijt R, Fidalgo P, Lage P, Slors FJ et al. (2002). The ‘just-right’ signaling model: APC somatic mutations are selected based on a specific level of activation of the beta-catenin signaling cascade. Hum Mol Genet 11: 1549–1560.
Aoki K, Taketo MM . (2007). Adenomatous polyposis coli (APC): a multi-functional tumor suppressor gene. J Cell Sci 120: 3327–3335.
Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M et al. (2009). Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 457: 608–611.
Behrens J, Jerchow BA, Wurtele M, Grimm J, Asbrand C, Wirtz R et al. (1998). Functional interaction of an axin homolog, conductin, with beta-catenin, APC, and GSK3beta. Science 280: 596–599.
Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R et al. (1996). Functional interaction of beta-catenin with the transcription factor LEF-1. Nature 382: 638–642.
Clarke AR . (2006). Wnt signalling in the mouse intestine. Oncogene 25: 7512–7521.
Clevers H. . (2006). Wnt/beta-catenin signaling in development and disease. Cell 127: 469–480.
Crabtree M, Sieber OM, Lipton L, Hodgson SV, Lamlum H, Thomas HJ et al. (2003). Refining the relation between ‘first hits’ and ‘second hits’ at the APC locus: the ‘loose fit’ model and evidence for differences in somatic mutation spectra among patients. Oncogene 22: 4257–4265.
Eklof Spink K, Fridman SG, Weis WI . (2001). Molecular mechanisms of beta-catenin recognition by adenomatous polyposis coli revealed by the structure of an APC-beta-catenin complex. Embo J 20: 6203–6212.
Ferrarese A, Marin O, Bustos VH, Venerando A, Antonelli M, Allende JE et al. (2007). Chemical dissection of the APC Repeat 3 multistep phosphorylation by the concerted action of protein kinases CK1 and GSK3. Biochemistry 46: 11902–11910.
Fodde R, Brabletz T . (2007). Wnt/beta-catenin signaling in cancer stemness and malignant behavior. Curr Opin Cell Biol 19: 150–158.
Gaspar C, Franken P, Molenaar L, Breukel C, van der Valk M, Smits R et al. (2009). A targeted constitutive mutation in the APC tumor suppressor gene underlies mammary but not intestinal tumorigenesis. PLoS Genet 5: e1000547.
Groden J, Gelbert L, Thliveris A, Nelson L, Robertson M, Joslyn G et al. (1993). Mutational analysis of patients with adenomatous polyposis: identical inactivating mutations in unrelated individuals. Am J Hum Genet 52: 263–272.
Groden J, Thliveris A, Samowitz W, Carlson M, Gelbert L, Albertsen H et al. (1991). Identification and characterization of the familial adenomatous polyposis coli gene. Cell 66: 589–600.
Groves C, Lamlum H, Crabtree M, Williamson J, Taylor C, Bass S et al. (2002). Mutation cluster region, association between germline and somatic mutations and genotype-phenotype correlation in upper gastrointestinal familial adenomatous polyposis. Am J Pathol 160: 2055–2061.
Gupta PK, Sahota A, Boyadjiev SA, Bye S, Shao C, O'Neill JP et al. (1997). High frequency in vivo loss of heterozygosity is primarily a consequence of mitotic recombination. Cancer Res 57: 1188–1193.
Ha NC, Tonozuka T, Stamos JL, Choi HJ, Weis WI . (2004). Mechanism of phosphorylation-dependent binding of APC to beta-catenin and its role in beta-catenin degradation. Mol Cell 15: 511–521.
Haigis KM, Dove WF . (2003). A Robertsonian translocation suppresses a somatic recombination pathway to loss of heterozygosity. Nat Genet 33: 33–39.
Hong Y, Cervantes RB, Tichy E, Tischfield JA, Stambrook PJ . (2007). Protecting genomic integrity in somatic cells and embryonic stem cells. Mutat Res 614: 48–55.
Kawahara K, Morishita T, Nakamura T, Hamada F, Toyoshima K, Akiyama T . (2000). Down-regulation of beta-catenin by the colorectal tumor suppressor APC requires association with Axin and beta-catenin. J Biol Chem 275: 8369–8374.
Kimelman D, Xu W . (2006). beta-catenin destruction complex: insights and questions from a structural perspective. Oncogene 25: 7482–7491.
Kohler EM, Chandra SH, Behrens J, Schneikert J . (2009). Beta-catenin degradation mediated by the CID domain of APC provides a model for the selection of APC mutations in colorectal, desmoid and duodenal tumours. Hum Mol Genet 18: 213–226.
Kohler EM, Derungs A, Daum G, Behrens J, Schneikert J . (2008). Functional definition of the mutation cluster region of adenomatous polyposis coli in colorectal tumours. Hum Mol Genet 17: 1978–1987.
Kotiligam D, Lazar AJ, Pollock RE, Lev D . (2008). Desmoid tumor: a disease opportune for molecular insights. Histol Histopathol 23: 117–126.
Krieghoff E, Behrens J, Mayr B . (2006). Nucleo-cytoplasmic distribution of \{beta\}-catenin is re. J Cell Sci 119: 1453–1463.
Kuraguchi M, Ohene-Baah NY, Sonkin D, Bronson RT, Kucherlapati R . (2009). Genetic mechanisms in Apc-mediated mammary tumorigenesis. PLoS Genet 5: e1000367.
Lamlum H, Ilyas M, Rowan A, Clark S, Johnson V, Bell J et al. (1999). The type of somatic mutation at APC in familial adenomatous polyposis is determined by the site of the germline mutation: a new facet to Knudson's ‘two-hit’ hypothesis. Nat Med 5: 1071–1075.
Latchford A, Volikos E, Johnson V, Rogers P, Suraweera N, Tomlinson I et al. (2007). APC mutations in FAP-associated desmoid tumours are non-random but not ‘just right’. Hum Mol Genet 16: 78–82.
Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y et al. (2002). Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 108: 837–847.
Liu J, Xing Y, Hinds TR, Zheng J, Xu W . (2006). The third 20 amino acid repeat is the tightest binding site of APC for beta-catenin. J Mol Biol 360: 133–144.
McCartney BM, Nathke IS . (2008). Cell regulation by the Apc protein Apc as master regulator of epithelia. Curr Opin Cell Biol 20: 186–193.
Menendez M, Gonzalez S, Obrador-Hevia A, Dominguez A, Pujol MJ, Valls J et al. (2008). Functional characterization of the novel APC N1026S variant associated with attenuated familial adenomatous polyposis. Gastroenterology 134: 56–64.
Miyaki M, Konishi M, Kikuchi-Yanoshita R, Enomoto M, Igari T, Tanaka K et al. (1994). Characteristics of somatic mutation of the adenomatous polyposis coli gene in colorectal tumors. Cancer Res 54: 3011–3020.
Miyaki M, Yamaguchi T, Iijima T, Takahashi K, Matsumoto H, Yasutome M et al. (2008). Difference in characteristics of APC mutations between colonic and extracolonic tumors of FAP patients: variations with phenotype. Int J Cancer 122: 2491–2497.
Miyoshi Y, Nagase H, Ando H, Horii A, Ichii S, Nakatsuru S et al. (1992). Somatic mutations of the APC gene in colorectal tumors: mutation cluster region in the APC gene. Hum Mol Genet 1: 229–233.
Munemitsu S, Albert I, Souza B, Rubinfeld B, Polakis P . (1995). Regulation of intracellular beta-catenin levels by the adenomatous polyposis coli (APC) tumor-suppressor protein. Proc Natl Acad Sci USA 92: 3046–3050.
Nathke I. . (2006). Cytoskeleton out of the cupboard: colon cancer and cytoskeletal changes induced by loss of APC. Nat Rev Cancer 6: 967–974.
Olschwang S, Laurent-Puig P, Groden J, White R, Thomas G . (1993). Germ-line mutations in the first 14 exons of the adenomatous polyposis coli (APC) gene. Am J Hum Genet 52: 273–279.
Peterson-Nedry W, Erdeniz N, Kremer S, Yu J, Baig-Lewis S, Wehrli M . (2008). Unexpectedly robust assembly of the Axin destruction complex regulates Wnt/Wg signaling in Drosophila as revealed by analysis in vivo. Dev Biol 320: 226–241.
Polakis P . (2007). The many ways of Wnt in cancer. Curr Opin Genet Dev 17: 45–51.
Pollard P, Deheragoda M, Segditsas S, Lewis A, Rowan A, Howarth K et al. (2009). The APC1322T mouse develops severe polyposis associated with submaximal nuclear beta-catenin expression. Gastroenterology 136: 2204–2213.
Powell SM, Zilz N, Beazer-Barclay Y, Bryan TM, Hamilton SR, Thibodeau SN et al. (1992). APC mutations occur early during colorectal tumorigenesis. Nature 359: 235–237.
Rowan AJ, Lamlum H, Ilyas M, Wheeler J, Straub J, Papadopoulou A et al. (2000). APC mutations in sporadic colorectal tumors: A mutational ‘hotspot’ and interdependence of the ‘two hits’. Proc Natl Acad Sci USA 97: 3352–3357.
Rubinfeld B, Albert I, Porfiri E, Munemitsu S, Polakis P . (1997). Loss of beta-catenin regulation by the APC tumor suppressor protein correlates with loss of structure due to common somatic mutations of the gene. Cancer Res 57: 4624–4630.
Rubinfeld B, Souza B, Albert I, Muller O, Chamberlain SH, Masiarz FR et al. (1993). Association of the APC gene product with beta-catenin. Science 262: 1731–1734.
Schneikert J, Behrens J . (2006). Truncated APC is required for cell proliferation and DNA replication. Int J Cancer 119: 74–79.
Schneikert J, Behrens J . (2007a). The canonical Wnt signalling pathway and its APC partner in colon cancer development. Gut 56: 417–425.
Schneikert J, Grohmann A, Behrens J . (2007b). Truncated APC regulates the transcriptional activity of beta-catenin in a cell cycle dependent manner. Hum Mol Genet 16: 199–209.
Segditsas S, Tomlinson I . (2006). Colorectal cancer and genetic alterations in the Wnt pathway. Oncogene 25: 7531–7537.
Smits R, Kielman MF, Breukel C, Zurcher C, Neufeld K, Jagmohan-Changur S et al. (1999). APC1638T: a mouse model delineating critical domains of the adenomatous polyposis coli protein involved in tumorigenesis and development. Genes Dev 13: 1309–1321.
Staal FJ, Noort Mv M, Strous GJ, Clevers HC . (2002). Wnt signals are transmitted through N-terminally dephosphorylated beta-catenin. EMBO Rep 3: 63–68.
Su LK, Vogelstein B, Kinzler KW . (1993). Association of the APC tumor suppressor protein with catenins. Science 262: 1734–1737.
Taketo MM . (2006). Wnt signaling and gastrointestinal tumorigenesis in mouse models. Oncogene 25: 7522–7530.
Tickenbrock L, Kossmeier K, Rehmann H, Herrmann C, Muller O . (2003). Differences between the interaction of beta-catenin with non-phosphorylated and single-mimicked phosphorylated 20-amino acid residue repeats of the APC protein. J Mol Biol 327: 359–367.
van de Wetering M, Cavallo R, Dooijes D, van Beest M, van Es J, Loureiro J et al. (1997). Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell 88: 789–799.
Xing Y, Clemens WK, Kimelman D, Xu W . (2003). Crystal structure of a beta-catenin/axin complex suggests a mechanism for the beta-catenin destruction complex. Genes Dev 17: 2753–2764.
Xing Y, Clements WK, Le Trong I, Hinds TR, Stenkamp R, Kimelman D et al. (2004). Crystal structure of a beta-catenin/APC complex reveals a critical role for APC phosphorylation in APC function. Mol Cell 15: 523–533.
Acknowledgements
We thank G Daum for technical, and A Doebler for secretarial assistances. We thank also I Wacker for critical reading of the paper. This work was supported by grants from the Interdisziplinäres Zentrum für Klinische Forschung (IZKF) and the Sander Stiftung to JB.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
Supplementary information
Rights and permissions
About this article
Cite this article
Kohler, E., Brauburger, K., Behrens, J. et al. Contribution of the 15 amino acid repeats of truncated APC to β-catenin degradation and selection of APC mutations in colorectal tumours from FAP patients. Oncogene 29, 1663–1671 (2010). https://doi.org/10.1038/onc.2009.447
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2009.447
Keywords
This article is cited by
-
Wnt secretion is required to maintain high levels of Wnt activity in colon cancer cells
Nature Communications (2013)
-
The value of epigenetic markers in esophageal cancer
Frontiers of Medicine in China (2010)