Abstract
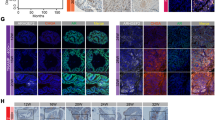
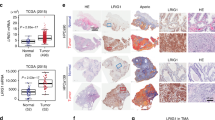
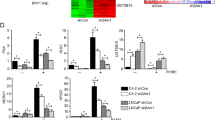
GATA-2, a member of the GATA family of transcription factors, is involved in androgen receptor (AR) signaling, however, little is known regarding its role in prostate cancer. Here, we report that GATA-2 is expressed in a substantial proportion of prostate cancers and that high expression of GATA-2 is associated with biochemical recurrence and distant metastatic progression in a validation set of 203 cancers. In vitro data show that GATA-2 is directly recruited to the promoter region of the AR upon androgen stimulation of LNCaP prostate cancer cells with 5α-dihydroxytestosterone (DHT) for 24 h. Ectopic GATA-2 expression causes the induction of AR transcript levels under androgen-depleted conditions (P<0.05). The expression of the AR target gene, AZGP1, is induced upon androgen stimulation and this effect is repressed by GATA-2. In contrast, GATA-2 significantly increases transcript levels of KLK2, which increases further in a time-dependent manner on DHT treatment and in the presence of GATA-2. These results indicate that upregulation of GATA-2 may contribute to the progression to aggressive prostate cancer through modulation of expression of AR and key androgen-regulated genes, one of which, AZGP1, is associated with the progression to metastatic disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bao Y, Bing C, Hunter L, Jenkins JR, Wabitsch M, Trayhurn P . (2005). Zinc-alpha2-glycoprotein, a lipid mobilizing factor, is expressed and secreted by human (SGBS) adipocytes. FEBS Lett 579: 41–47.
Bing C, Bao Y, Jenkins J, Sanders P, Manieri M, Cinti S et al. (2004). Zinc-alpha2-glycoprotein, a lipid mobilizing factor, is expressed in adipocytes and is up-regulated in mice with cancer cachexia. Proc Natl Acad Sci USA 101: 2500–2505.
Borgono CA, Michael IP, Diamandis EP . (2004). Human tissue kallikreins: physiologic roles and applications in cancer. Mol Cancer Res 2: 257–280.
Cox DR . (1972). Regression models and life-tables. J R Stat Soc Series B Methodol 34: 187–220.
Dehm SM, Tindall DJ . (2006). Molecular regulation of androgen action in prostate cancer. J Cell Biochem 99: 333–344.
Eeckhoute J, Keeton EK, Lupien M, Krum SA, Carroll JS, Brown M . (2007). Positive cross-regulatory loop ties GATA-3 to estrogen receptor alpha expression in breast cancer. Cancer Res 67: 6477–6483.
Genomatix; www.genomatix.de.
Hayakawa F, Towatari M, Ozawa Y, Tomita A, Privalsky ML, Saito H . (2004). Functional regulation of GATA-2 by acetylation. J Leukoc Biol 75: 529–540.
Heemers HV, Regan KM, Schmidt LJ, Anderson SK, Ballman KV, Tindall DJ . (2009). Androgen modulation of coregulator expression in prostate cancer cells. Mol Endocrinol 23: 572–583.
Hendriksen PJ, Dits NF, Kokame K, Veldhoven A, van Weerden WM, Bangma CH et al. (2006). Evolution of the androgen receptor pathway during progression of prostate cancer. Cancer Res 66: 5012–5020.
Henshall SM, Afar DE, Hiller J, Horvath LG, Quinn DI, Rasiah KK et al. (2003). Survival analysis of genome-wide gene expression profiles of prostate cancers identifies new prognostic targets of disease relapse. Cancer Res 63: 4196–4203.
Henshall SM, Horvath LG, Quinn DI, Eggleton SA, Grygiel JJ, Stricker PD et al. (2006). Zinc-alpha2-glycoprotein expression as a predictor of metastatic prostate cancer following radical prostatectomy. J Natl Cancer Inst 98: 1420–1424.
Horoszewicz JS, Leong SS, Chu TM, Wajsman ZL, Friedman M, Papsidero L et al. (1980). The LNCaP cell line—a new model for studies on human prostatic carcinoma. Prog Clin Biol Res 37: 115–132.
Ingenuity; http://www.ingenuity.com/index.html.
Kaplan EL, Meier P . (1958). Nonparametric Estimation from Incomplete Observations. J Am Stat Assoc 53: 457–481.
Kato S, Matsumoto T, Kawano H, Sato T, Takeyama K . (2004). Function of androgen receptor in gene regulations. J Steroid Biochem Mol Biol 89-90: 627–633.
Lin B, Ferguson C, White JT, Wang S, Vessella R, True LD et al. (1999). Prostate-localized and androgen-regulated expression of the membrane-bound serine protease TMPRSS2. Cancer Res 59: 4180–4184.
Lowry JA, Atchley WR . (2000). Molecular evolution of the GATA family of transcription factors: conservation within the DNA-binding domain. J Mol Evol 50: 103–115.
Magklara A, Smith CL . (2009). A composite intronic element directs dynamic binding of the progesterone receptor and GATA-2. Mol Endocrinol 23: 61–73.
Masuda K, Werner T, Maheshwari S, Frisch M, Oh S, Petrovics G et al. (2005). Androgen receptor binding sites identified by a GREF_GATA model. J Mol Biol 353: 763–771.
Matsuyama M, Yoshimura R . (2008). Peroxisome proliferator-activated receptor-gamma is a potent target for prevention and treatment in human prostate and testicular cancer. PPAR Res 2008: 249849.
Minegishi N, Suzuki N, Kawatani Y, Shimizu R, Yamamoto M . (2005). Rapid turnover of GATA-2 via ubiquitin-proteasome protein degradation pathway. Genes Cells 10: 693–704.
Mohler JL, Gregory CW, Ford 3rd OH, Kim D, Weaver CM, Petrusz P et al. (2004). The androgen axis in recurrent prostate cancer. Clin Cancer Res 10: 440–448.
Nelson PS, Clegg N, Arnold H, Ferguson C, Bonham M, White J et al. (2002). The program of androgen-responsive genes in neoplastic prostate epithelium. Proc Natl Acad Sci USA 99: 11890–11895.
Nemer G, Nemer M . (2003). Transcriptional activation of BMP-4 and regulation of mammalian organogenesis by GATA-4 and -6. Dev Biol 254: 131–148.
Oren T, Torregroza I, Evans T . (2005). An Oct-1 binding site mediates activation of the gata2 promoter by BMP signaling. Nucleic Acids Res 33: 4357–4367.
Perez-Stable CM, Pozas A, Roos BA . (2000). A role for GATA transcription factors in the androgen regulation of the prostate-specific antigen gene enhancer. Mol Cell Endocrinol 167: 43–53.
Rasiah KK, Kench JG, Gardiner-Garden M, Biankin AV, Golovsky D, Brenner PC et al. (2006). Aberrant neuropeptide Y and macrophage inhibitory cytokine-1 expression are early events in prostate cancer development and are associated with poor prognosis. Cancer Epidemiol Biomarkers Prev 15: 711–716.
Riegman PH, Vlietstra RJ, van der Korput JA, Brinkmann AO, Trapman J . (1991). The promoter of the prostate-specific antigen gene contains a functional androgen responsive element. Mol Endocrinol 5: 1921–1930.
Roche PJ, Hoare SA, Parker MG . (1992). A consensus DNA-binding site for the androgen receptor. Mol Endocrinol 6: 2229–2235.
Simon MC . (1995). Gotta have GATA. Nat Genet 11: 9–11.
Tong Q, Dalgin G, Xu H, Ting CN, Leiden JM, Hotamisligil GS . (2000). Function of GATA transcription factors in preadipocyte-adipocyte transition. Science 290: 134–138.
Usary J, Llaca V, Karaca G, Presswala S, Karaca M, He X et al. (2004). Mutation of GATA3 in human breast tumors. Oncogene 23: 7669–7678.
Wang G, Jones SJ, Marra MA, Sadar MD . (2006). Identification of genes targeted by the androgen and PKA signaling pathways in prostate cancer cells. Oncogene 25: 7311–7323.
Wang H, McKnight NC, Zhang T, Lu ML, Balk SP, Yuan X . (2007a). SOX9 is expressed in normal prostate basal cells and regulates androgen receptor expression in prostate cancer cells. Cancer Res 67: 528–536.
Wang Q, Carroll JS, Brown M . (2005). Spatial and temporal recruitment of androgen receptor and its coactivators involves chromosomal looping and polymerase tracking. Mol Cell 19: 631–642.
Wang Q, Li W, Liu XS, Carroll JS, Janne OA, Keeton EK et al. (2007b). A hierarchical network of transcription factors governs androgen receptor-dependent prostate cancer growth. Mol Cell 27: 380–392.
Weiss MJ, Orkin SH . (1995). GATA transcription factors: key regulators of hematopoiesis. Exp Hematol 23: 99–107.
Xu Y, Iyengar S, Roberts RL, Shappell SB, Peehl DM . (2003). Primary culture model of peroxisome proliferator-activated receptor gamma activity in prostate cancer cells. J Cell Physiol 196: 131–143.
Young CY, Andrews PE, Montgomery BT, Tindall DJ . (1992). Tissue-specific and hormonal regulation of human prostate-specific glandular kallikrein. Biochemistry 31: 818–824.
Acknowledgements
This work is supported by grants from the Cancer Institute NSW, the National Health and Medical Research Council, the Prostate Cancer Foundation of Australia and the RT Hall Trust. We thank Professor Liz Musgrove, Dr Ross Laybutt and Dr Liz Caldon for helpful discussion and critical reading of the paper. Furthermore, we thank Ruth PeBenito for technical support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
Rights and permissions
About this article
Cite this article
Böhm, M., Locke, W., Sutherland, R. et al. A role for GATA-2 in transition to an aggressive phenotype in prostate cancer through modulation of key androgen-regulated genes. Oncogene 28, 3847–3856 (2009). https://doi.org/10.1038/onc.2009.243
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2009.243
Keywords
This article is cited by
-
GATA2 co-opts TGFβ1/SMAD4 oncogenic signaling and inherited variants at 6q22 to modulate prostate cancer progression
Journal of Experimental & Clinical Cancer Research (2023)
-
Therapeutic targeting of FUBP3 phase separation by GATA2-AS1 inhibits malate-aspartate shuttle and neuroblastoma progression via modulating SUZ12 activity
Oncogene (2023)
-
Knockdown of estrogen receptor β increases proliferation and affects the transcriptome of endometrial adenocarcinoma cells
BMC Cancer (2019)
-
Expression and Function of Zinc-α2-Glycoprotein
Neuroscience Bulletin (2019)
-
The role of GATA2 in lethal prostate cancer aggressiveness
Nature Reviews Urology (2017)