Abstract
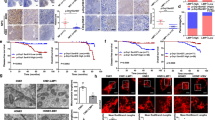
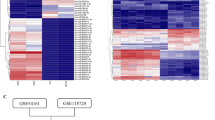
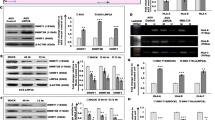
Tumor metastasis is a complex phenomenon that is the culmination of effects of numerous cellular factors. We have shown that the Epstein–Barr virus (EBV) oncoprotein, latent membrane protein 1 (LMP1), is capable of inducing a wide range of such factors in cell culture, expression of which is also elevated in the LMP1-expressing tumor, nasopharyngeal carcinoma (NPC), a highly invasive neoplasm. Recently, the membrane crosslinker protein, ezrin, has been implicated in tumor cell metastasis and malignant progression. In this study, we evaluated the possible role of LMP1 and ezrin in the pathophysiology of NPC. We show that C-terminal phosphorylation of ezrin is increased by the expression of LMP1 in nasopharyngeal (NP) cells through a protein kinase C (PKC) pathway. LMP1 enhances the organization of a ternary complex of CD44, ezrin and F-actin, which is a prerequisite for ezrin phosphorylation. In NPC tissues, the expression of phosphoezrin and LMP1 is directly correlated. Silencing of endogenously expressed ezrin suppresses LMP1-induced cell motility and invasiveness. Moreover, the inhibition of ezrin phosphorylation by PKC inhibitor suppresses migration and invasion of NP cells. These data show that the phosphorylation of ezrin and its recruitment to the cell membrane linked to F-actin and CD44 is a process required for LMP1-stimulated cell motility and invasion of NP cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Auvinen E, Kivi N, Vaheri A . (2007). Regulation of ezrin localization by Rac1 and PIPK in human epithelial cells. Exp Cell Res 313: 824–833.
Baumgartner M, Sillman AL, Blackwood EM, Srivastava J, Madson N, Schilling JW et al. (2006). The Nck-interacting kinase phosphorylates ERM proteins for formation of lamellipodium by growth factors. Proc Natl Acad Sci USA 103: 13391–13396.
Bruce B, Khanna G, Ren L, Landberg G, Jirström K, Powell C et al. (2007). Expression of the cytoskeleton linker protein ezrin in human cancers. Clin Exp Metastasis 24: 69–78.
Chuan YC, Pang ST, Cedazo-Minguez A, Norstedt G, Pousette A, Flores-Morales A . (2006). Androgen induction of prostate cancer cell invasion is mediated by ezrin. J Biol Chem 281: 29938–29948.
Contreras-Brodin BA, Anvret M, Imreh S, Altiok E, Klein G, Masucci MG . (1991). B cell phenotype-dependent expression of the Epstein–Barr virus nuclear antigens EBNA-2 to EBNA-6: studies with somatic cell hybrids. J Gen Virol 72: 3025–3033.
Crepaldi T, Gautreau A, Comoglio PM, Louvard D, Arpin M . (1997). Ezrin is an effector of hepatocyte growth factor-mediated migration and morphogenesis in epithelial cells. J Cell Biol 138: 423–434.
Doi Y, Itoh M, Yonemura S, Ishihara S, Takano H, Noda T et al. (1999). Normal development of mice and unimpaired cell adhesion/cell motility/actin-based cytoskeleton without compensatory up-regulation of ezrin or radixin in moesin gene knockout. J Biol Chem 274: 2315–2321.
Fais S . (2004). A role for ezrin in a neglected metastatic tumor function. Trends Mol Med 10: 249–250.
Fievet BT, Gautreau A, Roy C, Del Maestro L, Mangeat P, Louvard D et al. (2004). Phosphoinositide binding and phosphorylation act sequentially in the activation mechanism of ezrin. J Cell Biol 164: 653–659.
Gautreau A, Louvard D, Arpin M . (2002). ERM proteins and NF2 tumor suppressor: the Yin and Yang of cortical actin organization and cell growth signaling. Curr Opin Cell Biol 14: 104–109.
Hiscox S, Jiang WG . (1999). Ezrin regulates cell–cell and cell–matrix adhesion, a possible role with E-cadherin/beta-catenin. J Cell Sci 112: 3081–3090.
Hopkins AM, Pineda AA, Winfree LM, Brown GT, Laukoetter MG, Nusrat A . (2007). Organized migration of epithelial cells requires control of adhesion and protrusion through Rho kinase effectors. Am J Physiol Gastrointest Liver Physiol 292: G806–G817.
Horikawa T, Sheen TS, Takeshita H, Sato H, Furukawa M, Yoshizaki T . (2001). Induction of c-Met proto-oncogene by Epstein–Barr virus latent membrane protein-1 and the correlation with cervical lymph node metastasis of nasopharyngeal carcinoma. Am J Pathol 59: 27–33.
Horikawa T, Yang J, Kondo S, Yoshizaki T, Joab I, Furukawa M et al. (2007). Twist and epithelial–mesenchymal transition are induced by the EBV oncoprotein latent membrane protein 1 and are associated with metastatic nasopharyngeal carcinoma. Cancer Res 67: 1970–1978.
Khanna C, Wan X, Bose S, Cassaday R, Olomu O, Mendoza A et al. (2004). The membrane-cytoskeleton linker ezrin is necessary for osteosarcoma metastasis. Nat Med 10: 182–186.
Kondo S, Seo SY, Yoshizaki T, Wakisaka N, Furukawa M, Joab I et al. (2006). EBV latent membrane protein 1 up-regulates hypoxia-inducible factor 1alpha through Siah1-mediated down-regulation of prolyl hydroxylases 1 and 3 in nasopharyngeal epithelial cells. Cancer Res 66: 9870–9877.
Kondo S, Wakisaka N, Schell MJ, Horikawa T, Sheen TS, Sato H et al. (2005). Epstein–Barr virus latent membrane protein 1 induces the matrix metalloproteinase-1 promoter via an Ets binding site formed by a single nucleotide polymorphism: enhanced susceptibility to nasopharyngeal carcinoma. Int J Cancer 115: 368–376.
Kondo S, Yoshizaki T, Wakisaka N, Horikawa T, Murono S, Jang KL et al. (2007). MUC1 induced by Epstein–Barr virus latent membrane protein 1 causes dissociation of the cell–matrix interaction and cellular invasiveness via STAT signaling. J Virol 81: 1554–1562.
Larsson C . (2006). Protein kinase C and the regulation of the actin cytoskeleton. Cell Signal 18: 276–284.
Legg JW, Lewis CA, Parsons M, Ng T, Isacke CM . (2002). A novel PKC-regulated mechanism controls CD44 ezrin association and directional cell motility. Nat Cell Biol 4: 399–407.
Lo KW, To KF, Huang DP . (2004). Focus on nasopharyngeal carcinoma. Cancer Cell 5: 423–428.
Louvet-Vallée S . (2000). ERM proteins: from cellular architecture to cell signaling. Biol Cell 92: 305–316.
Martin TA, Harrison G, Mansel RE, Jiang WG . (2003). The role of the CD44/ezrin complex in cancer metastasis. Crit Rev Oncol Hematol 46: 165–186.
Miller WE, Mosialos G, Kieff E, Raab-Traub N . (1997). Epstein–Barr virus LMP1 induction of the epidermal growth factor receptor is mediated through a TRAF signaling pathway distinct from NF-kappaB activation. J Virol 71: 586–594.
Murono S, Inoue H, Tanabe T, Joab I, Yoshizaki T, Furukawa M et al. (2001). Induction of cyclooxygenase-2 by Epstein–Barr virus latent membrane protein 1 is involved in vascular endothelial growth factor production in nasopharyngeal carcinoma cells. Proc Natl Acad Sci USA 98: 6905–6910.
Ng T, Parsons M, Hughes WE, Monypenny J, Zicha D, Gautreau A et al. (2001). Ezrin is a downstream effector of trafficking PKC–integrin complexes involved in the control of cell motility. Embo J 20: 2723–2741.
Orian-Rousseau V, Chen L, Sleeman JP, Herrlich P, Ponta H . (2002). CD44 is required for two consecutive steps in HGF/c-Met signaling. Genes Dev 16: 3074–3086.
Park JH, Faller DV . (2002). Epstein–Barr virus latent membrane protein-1 induction by histone deacetylase inhibitors mediates induction of intercellular adhesion molecule-1 expression and homotypic aggregation. Virology 303: 345–363.
Raab-Traub N, Flynn K, Klein C, Pizza G, De Vinci C, Occhiuzzi L et al. (1987). EBV associated malignancies. J Exp Pathol 3: 449–456.
Stewart SA, Dykxhoorn DM, Palliser D, Mizuno H, Yu EY, An DS et al. (2003). Lentivirus-delivered stable gene silencing by RNAi in primary cells. RNA 9: 493–501.
Sun XG, Rotenberg SA . (1999). Overexpression of protein kinase Calpha in MCF-10A human breast cells engenders dramatic alterations in morphology, proliferation, and motility. Cell Growth Differ 10: 343–352.
Takimoto T, Sato H, Ogura H, Tanaka S, Umeda R . (1988). The difference in tumorigenicity between Epstein–Barr virus (EBV) genome-positive and genome-negative epithelial hybrid cell lines derived from the human nasopharynx. Laryngoscope 98: 1334–1338.
Tran Quang C, Gautreau A, Arpin M, Treisman R . (2000). Ezrin function is required for ROCK-mediated fibroblast transformation by the Net and Dbl oncogenes. EMBO J 19: 4565–4576.
Tsao SW, Tramoutanis G, Dawson CW, Lo AK, Huang DP . (2002a). The significance of LMP1 expression in nasopharyngeal carcinoma. Semin Cancer Biol 12: 473–487.
Tsao SW, Wang X, Liu Y, Cheung YC, Feng H, Zheng Z et al. (2002b). Establishment of two immortalized nasopharyngeal epithelial cell lines using SV40 large T and HPV16E6/E7 viral oncogenes. Biochim Biophys Acta 1590: 150–158.
Wakisaka N, Kondo S, Yoshizaki T, Murono S, Furukawa M, Pagano JS . (2004). Epstein–Barr virus latent membrane protein 1 induces synthesis of hypoxia-inducible factor 1 alpha. Mol Cell Biol 24: 5223–5234.
Wakisaka N, Murono S, Yoshizaki T, Furukawa M, Pagano JS . (2002). Epstein–Barr virus latent membrane protein 1 induces and causes release of fibroblast growth factor-2. Cancer Res 62: 6337–6344.
Walter J, Schirrmacher V, Mosier D . (1995). Induction of CD44 expression by the Epstein–Barr virus latent membrane protein LMP1 is associated with lymphoma dissemination. Int J Cancer 61: 363–369.
Yan G, Luo W, Lu Z, Luo X, Li L, Liu S et al. (2007). Epstein–Barr virus latent membrane protein 1 mediates phosphorylation and nuclear translocation of annexin A2 by activating PKC pathway. Cell Signal 19: 341–348.
Yoshizaki T, Sato H, Furukawa M, Pagano JS . (1998). The expression of matrix metalloproteinase 9 is enhanced by Epstein–Barr virus latent membrane protein 1. Proc Natl Acad Sci USA 95: 3621–3626.
Yoshizaki T, Wakisaka N, Pagano JS . (2005). Epstein–Barr virus, invasion and metastasis. In: Erle S. Robertson (ed). Epstein–Barr Virus, chapter 12. Caister Academic Press: Norfolk, pp 171–196.
Zhang Y, Hu MY, Wu WZ, Wang ZJ, Zhou K, Zha XL et al. (2006). The membrane-cytoskeleton organizer ezrin is necessary for hepatocellular carcinoma cell growth and invasiveness. J Cancer Res Clin Oncol 132: 685–697.
Zheng H, Li LL, Hu DS, Deng XY, Cao Y . (2007). Role of Epstein–Barr virus encoded latent membrane protein 1 in the carcinogenesis of nasopharyngeal carcinoma. Cell Mol Immunol 4: 185–196.
Acknowledgements
We thank Sai Wah Tsao and Erik K Flemington for the NP69SV40T cell line and the Ad-AH cell line, respectively. We thank Drs Yojiro Kotake and Yuki Okada for helpful advice. This work was supported by NCI (CA 19014) (JSP) and the Nakayama Foundation for Human Science (Tokyo, Japan) (KE).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
Supplementary information
Rights and permissions
About this article
Cite this article
Endo, K., Kondo, S., Shackleford, J. et al. Phosphorylated ezrin is associated with EBV latent membrane protein 1 in nasopharyngeal carcinoma and induces cell migration. Oncogene 28, 1725–1735 (2009). https://doi.org/10.1038/onc.2009.20
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2009.20
Keywords
This article is cited by
-
Epstein-Barr virus infection: the micro and macro worlds
Virology Journal (2023)
-
CD44 cross-linking increases malignancy of breast cancer via upregulation of p-Moesin
Cancer Cell International (2020)
-
Progression of understanding for the role of Epstein-Barr virus and management of nasopharyngeal carcinoma
Cancer and Metastasis Reviews (2017)
-
The prognostic significance of tyrosine-protein phosphatase nonreceptor type 12 expression in nasopharyngeal carcinoma
Tumor Biology (2015)
-
The tumor marker Fascin is induced by the Epstein-Barr virus-encoded oncoprotein LMP1 via NF-κB in lymphocytes and contributes to their invasive migration
Cell Communication and Signaling (2014)