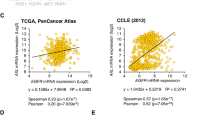
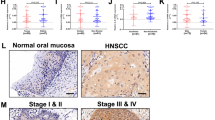
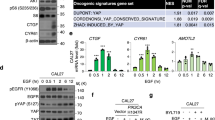
Abstract
Epidermal growth factor receptor (EGFR) tyrosine kinase is commonly overexpressed in human cancers; however, the cellular mechanisms regulating EGFR expression remain unclear. p53, p63 and p73 are transcription factors regulating many cellular targets involved in controlling the cell cycle and apoptosis. p53 activates EGFR expression, whereas TAp63 represses EGFR transcription. The involvement of p73 in the regulation of EGFR has not been reported. Here, a strong correlation between EGFR overexpression and increased levels of the oncogenic ΔNp73 isoform in head and neck squamous cell carcinoma (HNSCC) cell lines was observed. Ectopic expression of TAp73, particularly TAp73β, resulted in suppression of the EGFR promoter, significant downregulation of EGFR protein and efficient induction of cell death in all six EGFR-overexpressing HNSCC cell lines. EGFR overexpression from a heterologous LTR promoter protected lung cancer cells from TAp73β-induced EGFR suppression and apoptosis. Expression of TAp73β efficiently induced promyelocytic leukaemia (PML) protein expression and PML knockdown by shRNA attenuated the downregulation of EGFR and induction of apoptosis by p73 in HNSCC cells. Furthermore, PML was found to be important for E1A-induced suppression of EGFR and subsequent killing of HNSCC cells. Our data therefore suggest a novel pathway involving PML and p73 in the regulation of EGFR expression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Bergamaschi D, Gasco M, Hiller L, Sullivan A, Syed N, Trigiante G et al. (2003). p53 polymorphism influences response in cancer chemotherapy via modulation of p73-dependent apoptosis. Cancer Cell 3: 387–402.
Bernardi R, Pandolfi PP . (2003). Role of PML and the PML-nuclear body in the control of programmed cell death. Oncogene 22: 9048–9057.
Bernardi R, Papa A, Pandolfi PP . (2008). Regulation of apoptosis by PML and the PML-NBs. Oncogene 27: 6299–6312.
Bernassola F, Oberst A, Melino G, Pandolfi PP . (2005). The promyelocytic leukaemia protein tumour suppressor functions as a transcriptional regulator of p63. Oncogene 24: 6982–6986.
Bernassola F, Salomoni P, Oberst A, Di Como CJ, Pagano M, Melino G et al. (2004). Ubiquitin-dependent degradation of p73 is inhibited by PML. J Exp Med 199: 1545–1557.
Bischof O, Kirsh O, Pearson M, Itahana K, Pelicci PG, Dejean A . (2002). Deconstructing PML-induced premature senescence. EMBO J 21: 3358–3369.
Chen X, Zheng Y, Zhu J, Jiang J, Wang J . (2001). p73 is transcriptionally regulated by DNA damage, p53, and p73. Oncogene 20: 769–774.
de Melker AA, van der Horst G, Calafat J, Jansen H, Borst J . (2001). c-Cbl ubiquitinates the EGF receptor at the plasma membrane and remains receptor associated throughout the endocytic route. J Cell Sci 114: 2167–2178.
de Stanchina E, Querido E, Narita M, Davuluri RV, Pandolfi PP, Ferbeyre G et al. (2004). PML is a direct p53 target that modulates p53 effector functions. Mol Cell 13: 523–535.
de The H, Lavau C, Marchio A, Chomienne C, Degos L, Dejean A . (1991). The PML-RAR alpha fusion mRNA generated by the t(15;17) translocation in acute promyelocytic leukemia encodes a functionally altered RAR. Cell 66: 675–684.
Denizot A, Delfino C, Dutour-Meyer A, Fina F, Ouafik L . (2003). Evaluation of quantitative measurement of thyroglobulin mRNA in the follow-up of differentiated thyroid cancer. Thyroid 13: 867–872.
Flinterman M, Gaken J, Farzaneh F, Tavassoli M . (2003). E1A-mediated suppression of EGFR expression and induction of apoptosis in head and neck squamous carcinoma cell lines. Oncogene 22: 1965–1977.
Flinterman M, Guelen L, Ezzati-Nik S, Killick R, Melino G, Tominaga K et al. (2005). E1A activates transcription of p73 and Noxa to induce apoptosis. J Biol Chem 280: 5945–5959.
Flinterman MB, Mymryk JS, Klanrit P, Yousef AF, Lowe SW, Caldas C et al. (2007). p400 function is required for the adenovirus E1A-mediated suppression of EGFR and tumour cell killing. Oncogene 26: 6863–6874.
Fogal V, Gostissa M, Sandy P, Zacchi P, Sternsdorf T, Jensen K et al. (2000). Regulation of p53 activity in nuclear bodies by a specific PML isoform. EMBO J 19: 6185–6195.
Goddard AD, Borrow J, Freemont PS, Solomon E . (1991). Characterization of a zinc finger gene disrupted by the t(15;17) in acute promyelocytic leukemia. Science 254: 1371–1374.
Goldschneider D, Blanc E, Raguenez G, Barrois M, Legrand A, Le Roux G et al. (2004). Differential response of p53 target genes to p73 overexpression in SH-SY5Y neuroblastoma cell line. J Cell Sci 117: 293–301.
Guelen L, Paterson H, Gaken J, Meyers M, Farzaneh F, Tavassoli M . (2004). TAT-apoptin is efficiently delivered and induces apoptosis in cancer cells. Oncogene 23: 1153–1165.
Guo A, Salomoni P, Luo J, Shih A, Zhong S, Gu W et al. (2000). The function of PML in p53-dependent apoptosis. Nat Cell Biol 2: 730–736.
Gusterson BA, Anbazhagan R, Warren W, Midgely C, Lane DP, O’Hare M et al. (1991). Expression of p53 in premalignant and malignant squamous epithelium. Oncogene 6: 1785–1789.
Kakizuka A, Miller Jr WH., Umesono K, Warrell Jr RP, Frankel SR, Murty VV et al. (1991). Chromosomal translocation t(15;17) in human acute promyelocytic leukemia fuses RAR alpha with a novel putative transcription factor, PML. Cell 66: 663–674.
Kalyankrishna S, Grandis JR . (2006). Epidermal growth factor receptor biology in head and neck cancer. J Clin Oncol 24: 2666–2672.
Kastner P, Perez A, Lutz Y, Rochette-Egly C, Gaub MP, Chambon P . (1991). [Fusion proteins between PML and alpha-RAR in acute promyelocytic leukemia]. C R Seances Soc Biol Fil 185: 391–401.
Klanrit P, Flinterman MB, Odell EW, Melino G, Killick R, Norris JS et al. (2008). Specific isoforms of p73 control the induction of cell death induced by the viral proteins, E1A or apoptin. Cell Cycle 7: 205–215.
Lapi E, Di Agostino S, Donzelli S, Gal H, Domany E, Rechavi G et al. (2008). PML, YAP, and p73 are components of a proapoptotic autoregulatory feedback loop. Mol Cell 32: 803–814.
Lee CW, La Thangue NB . (1999). Promoter specificity and stability control of the p53-related protein p73. Oncogene 18: 4171–4181.
Ludes-Meyers JH, Subler MA, Shivakumar CV, Munoz RM, Jiang P, Bigger JE et al. (1996). Transcriptional activation of the human epidermal growth factor receptor promoter by human p53. Mol Cell Biol 16: 6009–6019.
Melino G, Bernassola F, Ranalli M, Yee K, Zong WX, Corazzari M et al. (2004). p73 induces apoptosis via PUMA transactivation and Bax mitochondrial translocation. J Biol Chem 279: 8076–8083.
Melino G, De Laurenzi V, Vousden KH . (2002). p73: friend or foe in tumorigenesis. Nat Rev Cancer 2: 605–615.
Nishi H, Senoo M, Nishi KH, Murphy B, Rikiyama T, Matsumura Y et al. (2001). p53 homologue p63 represses epidermal growth factor receptor expression. J Biol Chem 276: 41717–41724.
Pearson M, Carbone R, Sebastiani C, Cioce M, Fagioli M, Saito S et al. (2000). PML regulates p53 acetylation and premature senescence induced by oncogenic Ras. Nature 406: 207–210.
Prenzel N, Fischer OM, Streit S, Hart S, Ullrich A . (2001). The epidermal growth factor receptor family as a central element for cellular signal transduction and diversification. Endocr Relat Cancer 8: 11–31.
Racek T, Mise N, Li Z, Stoll A, Putzer BM . (2005). C-terminal p73 isoforms repress transcriptional activity of the human telomerase reverse transcriptase (hTERT) promoter. J Biol Chem 280: 40402–40405.
Sakai E, Tsuchida N . (1992). Most human squamous cell carcinomas in the oral cavity contain mutated p53 tumor-suppressor genes. Oncogene 7: 927–933.
Salomoni P, Pandolfi PP . (2002). The role of PML in tumor suppression. Cell 108: 165–170.
Sayan AE, Paradisi A, Vojtesek B, Knight RA, Melino G, Candi E. . (2005). New antibodies recognizing p73: comparison with commercial antibodies. Biochem Biophys Res Commun 330: 186–193.
Sheikh MS, Carrier F, Johnson AC, Ogdon SE, Fornace Jr AJ . (1997). Identification of an additional p53-responsive site in the human epidermal growth factor receptor gene promotor. Oncogene 15: 1095–1101.
Specht K, Richter T, Muller U, Walch A, Werner M, Hofler H . (2001). Quantitative gene expression analysis in microdissected archival formalin-fixed and paraffin-embedded tumor tissue. Am J Pathol 158: 419–429.
Tyteca S, Vandromme M, Legube G, Chevillard-Briet M, Trouche D . (2006). Tip60 and p400 are both required for UV-induced apoptosis but play antagonistic roles in cell cycle progression. EMBO J 25: 1680–1689.
Ueda Y, Hijikata M, Takagi S, Chiba T, Shimotohno K . (1999). New p73 variants with altered C-terminal structures have varied transcriptional activities. Oncogene 18: 4993–4998.
Vallian S, Chin KV, Chang KS . (1998). The promyelocytic leukemia protein interacts with Sp1 and inhibits its transactivation of the epidermal growth factor receptor promoter. Mol Cell Biol 18: 7147–7156.
Wang ZG, Delva L, Gaboli M, Rivi R, Giorgio M, Cordon-Cardo C et al. (1998). Role of PML in cell growth and the retinoic acid pathway. Science 279: 1547–1551.
Wu L, Zhu H, Nie L, Maki CG . (2004). A link between p73 transcriptional activity and p73 degradation. Oncogene 23: 4032–4036.
Wu Q, Hu H, Lan J, Emenari C, Wang Z, Chang KS et al. (2009). PML3 orchestrates the nuclear dynamics and function of TIP60. J Biol Chem 284: 8747–8759.
Wu WS, Vallian S, Seto E, Yang WM, Edmondson D, Roth S et al. (2001). The growth suppressor PML represses transcription by functionally and physically interacting with histone deacetylases. Mol Cell Biol 21: 2259–2268.
Yan DH, Chang LS, Hung MC . (1991). Repressed expression of the HER-2/c-erbB-2 proto-oncogene by the adenovirus E1a gene products. Oncogene 6: 343–345.
Yeudall WA, Jakus J, Ensley JF, Robbins KC . (1997). Functional characterization of p53 molecules expressed in human squamous cell carcinomas of the head and neck. Mol Carcinog 18: 89–96.
Yeudall WA, Paterson IC, Patel V, Prime SS . (1995). Presence of human papillomavirus sequences in tumour-derived human oral keratinocytes expressing mutant p53. Eur J Cancer B Oral Oncol 31B: 136–143.
Yokomizo A, Mai M, Tindall DJ, Cheng L, Bostwick DG, Naito S et al. (1999). Overexpression of the wild type p73 gene in human bladder cancer. Oncogene 18: 1629–1633.
Zaika AI, Slade N, Erster SH, Sansome C, Joseph TW, Pearl M et al. (2002). DeltaNp73, a dominant-negative inhibitor of wild-type p53 and TAp73, is up-regulated in human tumors. J Exp Med 196: 765–780.
Zhu J, Jiang J, Zhou W, Chen X . (1998). The potential tumor suppressor p73 differentially regulates cellular p53 target genes. Cancer Res 58: 5061–5065.
Acknowledgements
We thank Prof Bert Vogelstein for providing Ad-GFP-p53 and Ad-GFP, Prof Karen Vousden for providing Ad-GFP-TAp73β, Prof Alfred Johnson for providing pER1-luc and Associate Prof Kun-Sang Chang for anti-PML clone 3573 antibody. We also thank Prof Alan Lehmann for 1BR3 and 1BR3-LT, Prof Hugh Paterson for 6689, Prof Barry Gusterson for HN5, Prof Kazuya Tominaga for HSC-3 and HSC-3 M3, Prof Fiona Watts for Km3, Prof Andrew Yeudall for HN30 and Prof Stephen Prime for H103 and H357 cell lines. We especially thank Prof Christoph Borner for the critical and helpful reading of this paper. This work was supported by grants from Cancer Research UK, the Rosetrees Trust and Biomedical Research Centre at Guy's and St Thomas’ NHS Foundation Trust and King's College London. Marcella Flinterman was supported by a grant from Cancer Research UK (C1116). Poramaporn Klanrit and Patrayu Taebunpakul were supported by the Royal Thai Government Scholarship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
Rights and permissions
About this article
Cite this article
Klanrit, P., Taebunpakul, P., Flinterman, M. et al. PML involvement in the p73-mediated E1A-induced suppression of EGFR and induction of apoptosis in head and neck cancers. Oncogene 28, 3499–3512 (2009). https://doi.org/10.1038/onc.2009.191
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2009.191
Keywords
This article is cited by
-
Targeted therapy for head and neck cancer: signaling pathways and clinical studies
Signal Transduction and Targeted Therapy (2023)
-
Development of head and neck pathology in Europe
Virchows Archiv (2022)
-
Clinical update on head and neck cancer: molecular biology and ongoing challenges
Cell Death & Disease (2019)
-
A balancing act: orchestrating amino-truncated and full-length p73 variants as decisive factors in cancer progression
Oncogene (2015)
-
Functions, divergence and clinical value of TAp73 isoforms in cancer
Cancer and Metastasis Reviews (2013)