Abstract
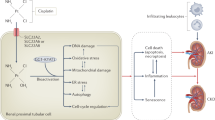
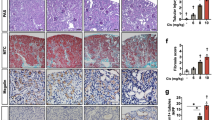
Cisplatin is one of the most effectively used chemotherapeutic agents for cancer treatment. However, in humans, important cytotoxic side effects are observed including dose-limiting renal damage and profound gastrointestinal symptomatology. The toxic responses to cisplatin in mice are similar to those in human patients. Here, we evaluated whether the acid sphingomyelinase (Asm) mediates at least some of the toxic in vivo effects of cisplatin. To this end, we determined the toxic effects of a single intraperitoneal dose of cisplatin (27 mg/kg) in wild type (Asm+/+) and Asm-deficient mice (Asm−/−). Tissue injury and apoptosis were determined histologically on hematoxylin–eosin and TUNEL (terminal deoxynucleotidyl transferase (TdT)-mediated nick end labeling) stainings 3, 12, 36 and 72 h after treatment. Our results revealed severe toxicity of cisplatin in Asm+/+ mice with increased numbers of apoptotic cells in the thymus and small intestine. In marked contrast, Asm−/− mice were resistant to cisplatin and no apoptosis was observed in these organs after treatment. Moreover, cisplatin treatment primarily triggered apoptosis of endothelial cells in microvessels of intestine and thymus, an effect that was absent in mice lacking Asm. The data thus suggest that at least some toxic effects of cisplatin are mediated by the Asm in vivo resulting in early death of endothelial cells and consecutive organ damage.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Allan SG, Smyth JF . (1986). Small intestinal mucosal toxicity of cis-platinum—comparison of toxicity with platinum analogues and dexamethasone. Br J Cancer 53: 355–360.
Bezombes C, Grazide S, Garret C, Fabre C, Quillet-Mary A, Muller S et al. (2004). Rituximab antiproliferative effect in B-lymphoma cells is associated with acid-sphingomyelinase activation in raft microdomains. Blood 104: 1166–1173.
Grunicke H, Hofmann J . (1992). Cytotoxic and cytostatic effects of antitumor agents induced at the plasma membrane level. Pharmacol Ther 55: 1–30.
Gulbins E, Grassme H . (2002). Ceramide and cell death receptor clustering. Biochim Biophys Acta 1585: 139–145.
Gulbins E, Kolesnick R . (2003). Raft ceramide in molecular medicine. Oncogene 22: 7070–7077.
Harrison Jr SD . (1981). Toxicologic evaluation of cis-diamminedichloroplatinum II in B6D2F1 mice. Fundam Appl Toxicol 1: 382–385.
Horinouchi K, Erlich S, Perl DP, Ferlinz K, Bisgaier CL, Sandhoff K et al. (1995). Acid sphingomyelinase deficient mice: a model of types A and B Niemann–Pick disease. Nat Genet 10: 288–293.
Huang C, Ma W, Ding M, Bowden GT, Dong Z . (1997). Direct evidence for an important role of sphingomyelinase in ultraviolet-induced activation of c-Jun N-terminal kinase. J Biol Chem 272: 27753–27757.
Lacour S, Hammann A, Grazide S, Lagadic-Gossmann D, Athias A, Sergent O et al. (2004). Cisplatin-induced CD95 redistribution into membrane lipid rafts of HT29 human colon cancer cells. Cancer Res 64: 3593–3598.
Loehrer PJ, Einhorn LH . (1984). Drugs five years later. Cisplatin. Ann Intern Med 100: 704–713.
Morita Y, Perez GI, Paris F, Miranda SR, Ehleiter D, Haimovitz-Friedman A et al. (2000). Oocyte apoptosis is suppressed by disruption of the acid sphingomyelinase gene or by sphingosine-1-phosphate therapy. Nat Med 6: 1109–1114.
Paris F, Fuks Z, Kang A, Capodieci P, Juan G, Ehleiter D et al. (2001). Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science 293: 293–297.
Pena LA, Fuks Z, Kolesnick RN . (2000). Radiation-induced apoptosis of endothelial cells in the murine central nervous system: protection by fibroblast growth factor and sphingomyelinase deficiency. Cancer Res 60: 321–327.
Prestayko AW, Bradner WT, Huftalen JB, Rose WC, Schurig JE, Cleare MJ et al. (1979). Antileukemia (L1210) activity and toxicity of cis-dichlorodiammineplatinum(II) analogs. Cancer Treat Rep 63: 1503–1508.
Rebillard A, Tekpli X, Meurette O, Sergent O, Le Moigne-Muller G, Vernhet L et al. (2007). Cisplatin-induced apoptosis involves membrane fluidification via inhibition of NHE1 in human colon cancer cells. Cancer Res 67: 7865–7874.
Sansom OJ, Clarke AR . (2002). The ability to engage enterocyte apoptosis does not predict long-term crypt survival in p53 and Msh2 deficient mice. Oncogene 21: 5934–5939.
Santana P, Pena LA, Haimovitz-Friedman A, Martin S, Green D, McLoughlin M et al. (1996). Acid sphingomyelinase-deficient human lymphoblasts and mice are defective in radiation-induced apoptosis. Cell 86: 189–199.
Tamaki T, Naomoto Y, Kimura S, Kawashima R, Shirakawa Y, Shigemitsu K et al. (2003). Apoptosis in normal tissues induced by anti-cancer drugs. J Int Med Res 31: 6–16.
Vasey PA, Jones NA, Jenkins S, Dive C, Brown R . (1996). Cisplatin, camptothecin, and taxol sensitivities of cells with p53-associated multidrug resistance. Mol Pharmacol 50: 1536–1540.
Acknowledgements
We thank F Paris, M Le Vée and I Guénon. This study was supported by grants from the Ligue Nationale Contre le Cancer (the Côte d'Armor, Ille et Vilaine and Loire-Atlantique Committees), Rennes Métropole, the Région Bretagne and DFG-10-2238-Gu2.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
Supplementary information
Rights and permissions
About this article
Cite this article
Rebillard, A., Rioux-Leclercq, N., Muller, C. et al. Acid sphingomyelinase deficiency protects from cisplatin-induced gastrointestinal damage. Oncogene 27, 6590–6595 (2008). https://doi.org/10.1038/onc.2008.257
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2008.257
Keywords
This article is cited by
-
A novel cisplatin mediated apoptosis pathway is associated with acid sphingomyelinase and FAS proapoptotic protein activation in ovarian cancer
Apoptosis (2015)
-
Specific disintegration of complex II succinate:ubiquinone oxidoreductase links pH changes to oxidative stress for apoptosis induction
Cell Death & Differentiation (2011)
-
Pleiotropic effects of amitriptyline ameliorate renal fibrosis
Kidney International (2009)