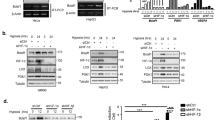
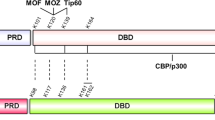
Abstract
The p53 tumour suppressor is involved in several crucial cellular functions including cell-cycle arrest and apoptosis. p53 stabilization occurs under hypoxic and DNA damage conditions. However, only in the latter scenario is stabilized p53 capable of inducing the expression of its pro-apoptotic targets. Here we present evidence that under hypoxia-mimicking conditions p53 acetylation is reduced to a greater extent at K320 site targeted by P300/CBP-associated factor (PCAF) than at K382 site targeted by p300/CBP. The limited amounts of acetylated p53 at K320 are preferentially recruited to the promoter of the p21WAF-1/CIP-1 gene, which appears to be unaffected by hypoxia, but are not recruited to the BID promoter and hence p53 is incapable of upregulating pro-apoptotic BID in hypoxic conditions. As the K320 p53 acetylation is the site predominantly affected in hypoxia, the PCAF histone acetyltransferase activity is the key regulator of the cellular fate modulated by p53 under these conditions. In addition, we provide evidence that PCAF acetylates hypoxia-inducible factor-1α (HIF-1α) in hypoxic conditions and that the acetylated HIF-1α is recruited to a particular subset of its targets. In conclusion, PCAF regulates the balance between cell-cycle arrest and apoptosis in hypoxia by modulating the activity and protein stability of both p53 and HIF-1α.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
An WG, Kanekal M, Simon MC, Maltepe E, Blagosklonny MV, Neckers LM . (1998). Stabilization of wild-type p53 by hypoxia-inducible factor 1α. Nature 392: 405–408.
Achison M, Hupp TP . (2003). Hypoxia attenuates the p53 response to cellular damage. Oncogene 22: 3431–3440.
Arany Z, Huang LE, Eckner R, Bhattacharya S, Jiang C, Goldberg MA et al. (1996). An essential role for p300/CBP in the cellular response to hypoxia. Proc Natl Acad Sci USA 93: 12969–12973.
Bilton R, Trottier E, Pouyssegur J, Brahimi-Horn MC . (2006). ARDent about acetylation and deacetylation in hypoxia signalling. Trends Cell Biol 16: 616–621.
Blagosklonny MV, An WG, Romanova LY, Trepel J, Fojo T, Neckers L . (1998). p53 inhibits hypoxia-inducible factor-stimulated transcription. J Biol Chem 273: 11995–11998.
Brahimi-Horn C, Mazure N, Pouysségur J . (2005). Signalling via the hypoxia-inducible factor-1α requires multiple posttranslational modifications. Cell Signalling 17: 1–9.
Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y et al. (2004). Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 303: 2011–2015.
Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown JP et al. (1998). Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science 282: 1497–1501.
Chao C, Wu Z, Mazur SJ, Borges H, Rossi M, Lin T et al. (2006). Acetylation of mouse p53 at lysine 317 negatively regulates p53 apoptotic activities after DNA damage. Mol Cell Biol 26: 6859–6869.
Chen D, Li M, Luo J, Gu W . (2003). Direct interactions between HIF-1α and Mdm2 modulate p53 function. J Biol Chem 278: 13595–13598.
Cohen HY, Lavu S, Bitterman KJ, Hekking B, Imahiyerobo TA, Miller C et al. (2004). Acetylation of the C terminus of Ku70 by CBP and PCAF controls Bax-mediated apoptosis. Mol Cell 13: 627–638.
Cory S, Huang DCS, Adams JM . (2003). The Bcl-2 family: roles in cell cycle survival and oncogenesis. Oncogene 22: 8590–8607.
Demonacos C, Krstic-Demonacos M, La Thangue NB . (2001). A novel TPR-motif co-factor contributes to p300 activity in the p53 response. Mol Cell 8: 71–84.
Demonacos C, Krstic-Demonacos M, Smith L, Xu D, O'Connor DP, Jansson M et al. (2004). A new effector pathway links ATM kinase with the DNA damage response. Nat Cell Biol 6: 968–976.
El-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM et al. (1993). WAF1, a potential mediator of p53 tumor suppression. Cell 75: 817–825.
Erler JT, Cawthorne CJ, Williams KJ, Koritzinksy M, Wouters B, Wilson C et al. (2004). Hypoxia mediated down regulation of Bid and Bax in tumours occurs via HIF-1 dependent and independent mechanisms and contributes to drug resistance. Mol Cell Biol 24: 2875–2889.
Fei P, Wang W, Kim S, Wang S, Burns TF, Sax JK et al. (2004). Bnip3L is induced by p53 under hypoxia, and its knockdown promotes tumor growth. Cancer Cell 6: 597–609.
Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD et al. (1996). Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol 16: 4604–4613.
Freedman SJ, Sun Z-YJ, Kung AL, France DS, Wagner G, Eck MJ . (2004). Structural basis for negative regulation of hypoxia-inducible factor-1α by CITED2. Nat Struct Biol 10: 504–512.
Freedman SJ, Sun Z-YJ, Poy F, Kung AL, Livingston DM, Wagner G et al. (2002). Structural basis for recruitment of CBP/p300 by hypoxia-inducible factor-1α. Proc Natl Acad Sci USA 99: 5367–5372.
Goda N, Dozier S, Johnson RS . (2003). HIF-1 in cell cycle regulation, apoptosis, and tumor progression. Antioxid Redox Signal 5: 467–473.
Gordan JD, Bertout JA, Hu C-J, Diehl JA, Simon MC . (2007). HIF-2a promotes hypoxic cell proliferation by enhancing c-Myc transcriptional activity. Cancer Cell 11: 335–347.
Greijer AE, van der Wall E . (2004). The role of hypoxia inducible factor 1 (HIF-1) in hypoxia induced apoptosis. J Clin Pathol 57: 1009–1014.
Hammond EM, Giaccia AJ . (2005). The role of p53 in hypoxia-induced apoptosis. Biochem Biophys Res Comm 331: 718–725.
Hammond EM, Mandell DJ, Salim A, Krieg AJ, Johnson TM, Shirazi HA et al. (2006). Genome-wide analysis of p53 under hypoxic conditions. Mol Cell Biol 26: 3492–3504.
Hansson LO, Friedler A, Freund S, Rudiger S, Fersht AR . (2002). Two sequence motifs from HIF-1α bind to the DNA-binding site of p53. Proc Natl Acad Sci USA 99: 10305–10309.
Hu CJ, Wang LY, Chodosh LA, Keith B, Simon MC . (2003). Differential roles of hypoxia inducible factor 1alpha (HIF-1alpha) and HIF-2alpha in hypoxic gene regulation. Mol Cell Biol 23: 9361–9374.
Jeong J-W, Bae M-K, Ahn M-Y, Kim S-H, Sohn T-K, Bae M-H et al. (2002). Regulation and destabilization of HIF-1α by ARD1-mediated acetylation. Cell 111: 709–720.
Jin Y, Zeng SX, Dai M-S, Yang X-J, Lu H . (2002). MDM2 Inhibits PCAF-mediated p53 acetylation. J Biol Chem 277: 30838–30843.
Kaelin Jr WG . (2005). Proline hydroxylation and gene expression. Annu Rev Biochem 74: 115–128.
Kasper LH, Boussouar F, Boyd K, Xu W, Biesen M, Rehg J et al. (2005). Two transactivation mechanisms cooperate for the bulk of HIF-1 responsive gene expression. EMBO J 24: 3846–3858.
Knights CD, Catania J, Di Giovani S, Muratoglu S, Perez R, Swartzbeck A et al. (2006). Distinct p53 acetylation cassettes differentially influence gene-expression patterns and cell fate. J Cell Biol 173: 533–544.
Koumenis C, Alarcon R, Hammond E, Sutphin P, Hoffman W, Murphy M et al. (2001). Regulation of p53 by hypoxia: dissociation of transcriptional repression and apoptosis from p53-dependent transactivation. Mol Cell Biol 21: 1297–1310.
Lando D, Peet DJ, Whelan DA, Gorman JJ, Whitelaw ML . (2002). Asparagine hydroxylation of the HIF transactivation domain: a hypoxic switch. Science 295: 858–861.
Liang D, Kong X, Sang N . (2006). Effects of histone deacetylase inhibitors on HIF-1. Cell Cycle 5: 2430–2435.
Linares LK, Kiernan R, Triboulet R, Chable-Bessia C, Latreille D, Cuvier O et al. (2007). Intrinsic ubiquitination activity of PCAF controls the stability of the oncoprotein Hdm2. Nat Cell Biol 9: 331–338.
Liu L, Scolnick DM, Trievel RC, Zhang HB, Marmorstein R, Halazonetis TD et al. (1999). p53 sites acetylated in vitro by PCAF and p300 are acetylated in vivo in response to DNA damage. Mol Cell Biol 19: 1202–1209.
Luo J, Li M, Tang Y, Laszkowska M, Roeder RG, Gu W . (2004). Acetylation of p53 augments its site-specific DNA binding both in vitro and in vivo. Proc Natl Acad Sci USA 101: 2259–2264.
Mahon PC, Hirota K, Semenza GL . (2001). FIH-1: a novel protein that interacts with HIF-1α and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev 15: 2675–2686.
Pan Y, Ogrysko PR, Arsham AM, Koch CJ, Simon MC . (2004). p53 cannot be induced by hypoxia alone but responds to the hypoxic microenvironment. Oncogene 23: 4975–4983.
Pescador N, Cuervas Y, Naranjo S, Alcaide M, Villar D, Landazuri MD et al. (2005). Identification of a functional hypoxia-responsive element that regulates the expression of egl nine homologue 3 (egln/phd3) gene. Biochem J 390: 189–197.
Poux AN, Marmorstein R . (2003). Molecular basis for Gcn5/PCAF histone acetyltransferase selectivity for histone and non-histone substrates. Biochemistry 42: 14366–14372.
Ravi R, Mookerjee B, Bhujwalla ZM, Sutter CH, Artemov D, Zeng Q et al. (2000). Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1α. Genes Dev 14: 34–44.
Raval RR, Lau KW, Tran MG, Sowter HM, Mandriota SJ, Li JL et al. (2005). Contrasting properties of hypoxia-inducible factor 1 (HIF-1) and HIF-2 in von-Hippel-Lindau-associated renal cell carcinoma. Mol Cell Biol 25: 5675–5686.
Roy S, Packman K, Jeffrey R, Tenniswood M . (2005). Histone deacetylase inhibitors differentially stabilise acetylated p53 and induce cell cycle arrest or apoptosis in prostate cancer cells. Cell Death Diff 12: 482–491.
Roy S, Tenniswood M . (2007). Site-specific acetylation of p53 directs selective transcription complex assembly. J Biol Chem 282: 4765–4771.
Sakaguchi KJ, Herrera S, Saito T, Miki M, Bustin A, Vassilev A et al. (1998). DNA damage activates p53 through a phosphorylation-acetylation cascade. Genes Dev 12: 2831–2841.
Sax JK, Fei P, Murphy ME, Bernhard E, Korsmeyer SJ, El-Deiry WS . (2002). BID regulation by ER contributes to chemosensitivity. Nat Cell Biol 4: 842–849.
Schmid T, Zhou J, Köhl R, Brüne B . (2004). p300 relieves p53-evoked transcriptional repression of hypoxia-inducible factor-1 (HIF-1). Biochem J 380: 289–295.
Semenza GL, Jiang B-H, Leung SW, Passantino R, Concordet J-P, Maire P et al. (1996). Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase a gene promoters contain essential binding sites for hypoxia-inducible factor 1. J Biol Chem 271: 32529–32537.
Tyteca S, Legube G, Trouche D . (2006). To die or not to die: A HAT trick. Mol Cell 24: 807–812.
Vaupel P, Harrison L . (2004). Tumor hypoxia: causative factors, compensatory mechanisms, and cellular response. Oncologist 9 (Suppl 5): 4–9.
Vousden KH, Lane DP . (2007). p53 in health and disease. Nat Rev Mol Cell Biol 8: 275–283.
Vousden KH, Prives C . (2005). p53 and prognosis; new insights and further complexity. Cell 120: 7–10.
Warnecke C, Zaborowska Z, Kurreck J, Erdmann VA, Frei U, Wiesener M et al. (2004). Differentiating the functional role of hypoxia-inducible factor (HIF)-1a and HIF-2a (EPAS-1) by the use of RNA interference: erythropoietin is a HIF-2alpha target gene in Hep3B and Kelly cells. FASEB J 18: 1462–1464.
Wenger RH . (2002). Cellular adaptation to hypoxia: O2-sensing protein hydroxylases, hypoxia-inducible transcription factors, and O2-regulated gene expression. FASEB J 16: 1151–1162.
Wykoff CC, Beasley NJP, Watson PH, Turner KJ, Pastorek J, Sibtain A et al. (2000). Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res 60: 7075–7083.
You H, Pellegrini M, Tsuchihara K, Yamamoto K, Hacker G, Erlacher M et al. (2006). FOXO3a-dependent regulation of Puma in response to cytokine/growth factor withdrawal. J Exp Med 203: 1657–1663.
Zhu Y, Mao XO, Sun Y, Xia Z, Greenberg DA . (2002). p38 mitogen-activated protein kinase mediates hypoxic regulation of Mdm2 and p53 in neurons. J Biol Chem 277: 22909–22914.
Acknowledgements
We thank W El-Deiry for providing the BID-p53-Luc reporter construct; I Talianidis for the Flag-PCAF and Flag-PCAF-ΔHAT constructs; T Halazonetis for the p53K320R expression vector; K Williams for the HIF-1α-responsive reporters of VEGF-Luc, CA-IX-Luc, PGK-1-Luc and LDH-A-Luc; M Blaylock and the Paterson Institute for Cancer Research for assistance with FACS analysis. Our research was supported by the School of Pharmacy, University of Manchester (CD), Cancer Research UK (CDive), MRC programme grant (G0500366) to IJS and Wellcome Trust (069024) to MKD.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
Supplementary information
Rights and permissions
About this article
Cite this article
Xenaki, G., Ontikatze, T., Rajendran, R. et al. PCAF is an HIF-1α cofactor that regulates p53 transcriptional activity in hypoxia. Oncogene 27, 5785–5796 (2008). https://doi.org/10.1038/onc.2008.192
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2008.192
Keywords
This article is cited by
-
p53 regulation by ubiquitin and ubiquitin-like modifications
Genome Instability & Disease (2022)
-
The role of epigenetic modifications in Colorectal Cancer Metastasis
Clinical & Experimental Metastasis (2022)
-
The role of hypoxic signalling in metastasis: towards translating knowledge of basic biology into novel anti-tumour strategies
Clinical & Experimental Metastasis (2018)
-
Epigenetic regulators: multifunctional proteins modulating hypoxia-inducible factor-α protein stability and activity
Cellular and Molecular Life Sciences (2018)
-
Preconditioning of primary human renal proximal tubular epithelial cells without tryptophan increases survival under hypoxia by inducing autophagy
International Urology and Nephrology (2017)