Abstract
BACKGROUND/OBJECTIVES:
Subjects with type-2 diabetes are typically obese with dysfunctional adipose tissue (AT). Glucagon-like peptide-1 (GLP-1) analogues are routinely used to improve glycaemia. Although, they also aid weight loss that improves AT function, their direct effect on AT function is unclear. To explore GLP-1 analogues’ influence on human AT’s cytokine and extracellular matrix (ECM) regulation, we therefore obtained and treated omental (OMAT) and subcutaneous (SCAT) AT samples with Exendin-4, an agonist of the GLP-1 receptor (GLP-1R).
SUBJECTS/METHODS:
OMAT and abdominal SCAT samples obtained from women during elective surgery at the Royal Devon & Exeter Hospital (UK) were treated with increasing doses of Exendin-4. Changes in RNA expression of adipokines, inflammatory cytokines, ECM components and their regulators were assessed and protein secretion analysed by ELISA. GLP-1R protein accumulation was compared in paired AT depot samples.
RESULTS:
Exendin-4 induced an increase in OMAT adiponectin (P=0.02) and decrease in elastin expression (P=0.03) in parallel with reduced elastin secretion (P=0.04). In contrast to OMAT, we did not observe an effect on SCAT. There was no change in the expression of inflammatory markers (CD14, TNFA, MCP-1), collagens, TGFB1 or CTGF. GLP-1R accumulation was higher in SCAT.
CONCLUSIONS:
Independently of weight loss, which may bias findings of in vivo studies, GLP-1 analogues modify human OMAT physiology favourably by increasing the insulin-sensitising cytokine adiponectin. However, the reduction of elastin and no apparent effect on AT’s inflammatory cytokines suggest that GLP-1 analogues may be less beneficial to AT function, especially if there is no associated weight loss.
Similar content being viewed by others
INTRODUCTION
Glucagon-like peptide-1 (GLP-1) is an incretin hormone produced by the intestinal L-cells from a post-translational processing of proglucagon.1 GLP-1 secretion is enhanced in response to nutrient ingestion and leads to a glucose-dependent increase of insulin release, which contributes to improved glucose homoeostasis. GLP-1 modulates satiety and reduces gastric emptying typically with a net effect of weight loss. GLP-1 has a short half-life owing to its rapid enzymatic degradation by dipeptidyl peptidase-4. Several analogues resistant to dipeptidyl peptidase-4 are routinely used for type-2 diabetes treatment. The concomitant weight loss also observed in non-diabetic patients led to consideration of GLP-1 analogues for obesity care.2
Adipose tissue (AT) is a connective tissue in which cells are embedded in a dense extracellular matrix (ECM) composed of structural proteins (collagens, elastin) and adhesion proteins (fibronectin, proteoglycans and so on), which ensure its mechanical stability, strength and elasticity.3 Obesity is associated with profound remodelling of AT’s ECM that can lead to the establishment of fibrosis.4 Together with chronic inflammation, macrophage infiltration and adipocyte hypertrophy, these changes characterise AT dysfunction of obesity and insulin resistance. It is well established that inflammation as one of the key features of AT dysfunction improves with weight loss,5 whereas direct GLP-1 effects on human AT physiology are poorly defined and difficult to distinguish, in vivo, from GLP-1-induced weight loss and related improvement in glucose control as result of its incretin effect. Its receptor, GLP-1 receptor (GLP-1R), is expressed in human AT, especially by cells of the stromal vascular fraction but also by adipocytes,6 suggesting a role for GLP-1 on AT. Beneficial effects of GLP-1 were reported on modulation of ECM remodelling in mice myocardium showing that Exendin-4 may be able to protect from post-myocardial infarction associated interstitial fibrosis by limiting inflammation and reducing transforming growth factor β3 (TGFβ3) and collagen expression,7 however, the direct effects of GLP-1 on AT, especially in view of inflammation and ECM remodelling have not yet been examined.
In order to understand whether GLP-1 analogues improve AT dysfunction independent of weight loss and, especially as not all patients treated with GLP-1 analogues lose weight, the present study aims to evaluate potential effects of Exendin-4, a GLP-1 analogue, on human AT physiology in omental (OMAT) and subcutaneous AT (SCAT) explants.
MATERIALS AND METHODS
AT collection
OMAT and SCAT biopsies were obtained with consent from participants undergoing elective abdominal surgery at the Royal Devon & Exeter Hospital with ethics permission granted by the Royal Devon & Exeter Tissue Bank Steering Committee of the Exeter NIHR Clinical Research Facility (Exeter, UK). AT samples for explant culture were collected from seven non-diabetic overweight women with full ethical consent (seven subcutaneous abdominal with six paired OMAT biopsies). In brief, the patient characteristics of each group from this cohort were: OMAT (mean±s.d.): n=6, age 60.2±11.2 years, BMI 29.0±6.9 kg m−2, subcutaneous: n=7, age 60.7±10.3 years, BMI 28.4±6.5 kg m−2. AT samples used for protein extraction were paired OMAT and SCAT biopsies collected from eight women of which five were diagnosed with diabetes; age 51.4±6.5 years, BMI 44.6±7.8 kg m−2. Subjects with acute or chronic inflammatory disease, those that had undergone steroid treatment in the last 3 months or had recently taken part in a weight loss intervention/surgery were excluded.
AT explant culture
AT was collected in Hank's Balanced Salt Solution (PAA laboratories, Pasching, Austria) supplemented with 1% 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (Gibco, Life Technologies, Paisley, UK), 0.1% Gentamicin, 1% Amphotericin (both from Sigma-Aldrich, St Louis, MO, USA). Tissue was dissected into 5–10 mg pieces, removing blood vessels and connective tissue. Approximately 250 mg of tissue was cultured in Media 199 (Gibco, Life Technologies), supplemented with 5% fetal calf serum and 1% penicillin/streptomycin) with increasing concentrations of Exendin-4 (Isca Biochemicals, Exeter, UK), a dipeptidyl peptidase-4-resistant GLP-1R agonist sharing 53% structural homology with GLP-1.8 Tissue explants were incubated at 37 °C for 45 h in an atmosphere of 5% CO2. Media was used for analysis of protein secretion by ELISA and tissue frozen in Tri Reagent solution (Ambion, Life Technologies) for further RNA processing.
RNA extraction
RNA was extracted using TRI Reagent according to the manufacturer’s protocol. Isolated RNA was treated with DNase I (Thermo Scientific, Paisley, UK) and converted to complementary DNA using the SuperScript VILO cDNA Synthesis Kit (Invitrogen, Life Technologies) as previously described.9
Gene expression analysis
Gene expression analysis was carried out using Taqman Low-Density Array cards (Life Technologies) on an ABI7900HT instrument (Applied Biosystems, Life Technologies). Relative gene expression was determined using the ΔΔCT method, with expression levels normalised to the geometric mean of three housekeeping genes (GAPDH, PPIA and UBC). TaqMan probes used are listed in Supplementary Table 1. All samples were amplified in duplicate and data are presented as arbitrary units.
Western blot
Tissue samples were homogenised in radioimmunoprecipitation assay buffer as previously described.10 Total protein (30 μg) was separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis. Primary antibodies used were GLP-1 R (1:500, ab186051, Abcam, Cambridge, UK) and β-actin (1:1000, A5441, Sigma-Aldrich, Gillingham, UK). Infrared fluorescent signals were detected with relevant secondary antibodies (Li-Cor, Lincoln, NE, USA) using an Odyssey CLx and quantified with Image studio (Li-Cor).
ELISA
Protein release in supernatant was evaluated using ELISA kits: total adiponectin, monocyte chemoattractant protein-1 (MCP-1) (both from Life Technologies) and elastin (Cloud-Clone Corp., Caltag MedSystems Ltd, Buckingham, UK). Samples were run in duplicate and absorbance measured at 450nm wavelength using a PHERAstar FS (BMG Labtech, Aylesbury, UK) microplate reader.
Statistical analysis
All data are presented as the mean±s.e.m. unless otherwise stated. Assessment of the dose response of Exendin-4 was analysed using Friedman’s one-way analysis of variance followed by a Dunn’s post hoc test to correct for multiple testing. For western blot analysis, statistical significance was assessed using a Wilcoxon test. Statistics were performed using GraphPad Prism 5.04 software (La Jolla, CA, USA), P-values<0.05 were considered significant.
RESULTS
In the following sections, we studied the effect of GLP-1R agonists on human OMAT and SCAT by studying the dose response to Exendin-4 treatment ex vivo.
Adipokines
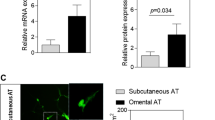
Adiponectin (ADIPOQ) exerts cardioprotective, insulin-sensitising and anti-inflammatory effects.11 AT explant treatment with Exendin-4 increased ADIPOQ expression in OMAT (Figure 1a and Table 1) with no effect in SCAT (Table 1). To confirm this observation, we measured adiponectin levels in supernatant obtained from explant culture. We did not observe any modification of adiponectin secretion in OMAT or SCAT (Figure 1b). Leptin is involved in the regulation of satiety, energy expenditure, body weight and insulin sensitivity.11 LEP expression was not significantly altered by Exendin-4 in either OMAT or SCAT explants (Table 1).
Exendin-4 stimulated adiponectin expression in OMAT explants. (a) Omental (OMAT) AT’s adiponectin mRNA expression as result of increasing doses of Exendin-4 (0–100 nm). (b). Adiponectin concentrations in supernatant obtained from culture of OMAT and subcutaneous adipose tissue (SCAT) explants with Exendin-4. *P<0.05, OMAT, n=6; SCAT, n=7.
Inflammatory cytokines
Monocyte chemoattractant protein-1 (MCP-1) and tumour necrosis factor alpha (TNFα) are produced by both adipocytes and AT resident macrophages and CD14 (cluster of differentiation 14) is a monocyte/macrophage marker expression of which reflects macrophage infiltration in AT.12 MCP-1 (also called CCL2; CC-chemokine ligand 2) is involved in immune cell recruitment into AT. Its expression was not significantly affected by increasing doses of Exendin-4 in either OMAT, or SCAT. We confirmed this observation by analysis of its protein secretion in the explant media and found no change (data not shown). Similarly, we did not find any changes in TNFA or CD14 expression in response to Exendin-4 (see Table 1). We also did not find any modification in the expression of inflammatory markers after treatment with Insulin with or without Exendin-4 (data not shown).
Fibrosis: ECM and its regulators
In order to assess Exendin-4’s impact on AT’s ECM composition, we analysed expression of ECM main proteins (collagens, fibronectin and elastin), enzymes involved in their cross-linking (LOX, lysosyl oxidase and LOXL2, lysyl oxidase-like 2) and degradation (MMPs, metalloproteinases) as well as regulators of ECM remodelling (TGFβ1, transforming growth factor beta 1 and CTGF, connective tissue growth factor). We analysed expression of the three main collagen types found in AT: COL1A1 (collagen 1 alpha subunit 1), COL3A1 (collagen 3 alpha subunit 1) and the pericellular COL4A1 (collagen 4 alpha subunit 1).9 We did not find any significant changes in the expression of these collagen subunits in OMAT or SCAT (Table 1). Similarly, fibronectin (FN1) did not appear to be affected by Exendin-4 treatment. In contrast to the collagens, elastin fibres provide stretchiness to AT.3 We found that Exendin-4 treatment significantly decreased elastin (ELN) expression within OMAT explants, however, this effect was not seen within SCAT. We therefore assessed elastin synthesis by measuring its release in culture media obtained from AT explants treated with Exendin-4. Exposure of AT explants to high dose of Exendin-4 significantly decreased elastin synthesis in OMAT explants but not SCAT (Figure 2b).
Exendin-4 treatment decreased expression and secretion of elastin in OMAT. (a) Accumulation of elastin mRNA in OMAT after treatment with 0, 1, 10, 100 nM of Exendin-4 (OMAT, n=6; SCAT, n=7). (b) Elastin release into media of OMAT and SCAT in response to Exendin-4 treatments and significant for comparison of 0 nm vs 100 nM (OMAT, n=9; SCAT, n=7). *P<0.05.
LOX and LOXL2 are two enzymes involved in collagen and elastin cross-linking.13 We found that neither LOX nor LOXL2 responded to Exendin-4. Similarly, we observed no change on expression of two key enzymes involved in ECM break down: MMP9 and MMP14. Moreover in response to Exendin-4 treatment, we monitored no change in the two pro-fibrotic messengers: TGFB1 and CTGF (Table 1).
Other proteins relevant to AT function
We assessed the expression of PPARG (peroxisome proliferator-activated receptor gamma), one of the main regulator of adipogenesis and did not observe any modification, nor did we find a change in the hypoxia marker HIF1A (hypoxia inducible factor 1 alpha) with hypoxia being one of the mediators of fibrosis and inflammation.14 Also, there were no changes in the lipogenesis regulator LPL (lipoprotein lipase) or the endothelial factor CD31 (cluster of differentiation 31)/PECAM1 (platelet/endothelial cell adhesion molecule 1, see Table 1).
GLP-1R expression in OMAT and SCAT
Owing to the marked differences in the depot response to Exendin-4 of both adiponectin and elastin, we further examined whether this could be due to a difference in receptor expression. Using a cohort of paired OMAT and SCAT samples (n=8), we observed, by western blot, a stronger accumulation of GLP-1R in SCAT (Figure 3).
DISCUSSION
We examined direct effects of GLP-1 analogues on abdominal AT depots, which are prone to expand with increasing obesity and susceptible to AT dysfunction.15 Our study shows that acute treatments based on GLP-1 analogues may have an advantage by increasing adiponectin expression and that they reduce elastin secretion by which they take part in ECM remodelling. Studying whole AT, with its stromal vascular fraction, enables to account for the cross-talk between its different cell populations. Preadipocytes and adipocytes both express GLP-1R6 and are involved in ECM protein production. This model allowed us for the first time to study effects of GLP-1 on the ECM without the influence of weight loss or glycaemic improvement which are recognized effects of GLP-1 analogue treatments.
GLP-1 and adiponectin
In response to Exendin-4, we found a dose-dependent increase of adiponectin expression in OMAT explants. Adiponectin is, mainly secreted by adipocytes, with plasma concentrations inversely correlated with insulin resistance, type-2 diabetes and obesity.11 Adiponectin has insulin-sensitising and anti-inflammatory properties and an ability to enhance pancreatic β-cells regeneration.16 Though adiponectin is mainly secreted by SCAT, Drolet et al.17 found that the OMAT release of adiponectin was reduced with increasing BMI, total body fat mass and visceral AT area, suggesting that OMAT could be responsible for the hypoadiponectinemia associated with obesity. Treatments of patients with GLP-1R agonists promote weight loss and increase circulating adiponectin levels.18 However, little is so far known about GLP-1 treatment effects on human AT in vivo controlled for weight change, apart from a recent study in which Exenatide was started in type-2 diabetic subjects prior to bariatric surgery. This study has shown similar to us, a selectively increase of adiponectin expression in visceral not SCAT.19 In line with these findings, our results go further and show that Exendin-4 treatment changes adiponectin expression in OMAT has yet little effect on adiponectin secretion. Thus, the increased circulating adiponectin secretion as previously reported with GLP-1 treatment is mainly a feature of weight loss.20 An accumulative effect, stronger with chronic treatment, cannot be excluded. Murine studies postulate activation of a PKA-dependent pathway21 and M2 macrophage polarisation22 as potential pathways by which GLP-1 may stimulate adiponectin expression.
GLP-1 and ECM
Weight loss is accompanied by profound ECM remodelling. In vitro studies allow assessment of GLP-1 effects independent of weight loss. We showed a decrease of elastin expression in OMAT in response to Exendin-4, whereas fibre forming type I, II and IV collagens did not appear to be affected. Elastin confers resilience, elasticity and deformability to many connective tissues including AT.3 Elastin was reported to be less abundant in AT from non-diabetic obese than lean subjects when assessed by immunohistology.23 Furthermore, mice with haplo-insufficiency of the elastin gene in a ApoE−/− background had impaired glucose metabolism and demonstrated adipocyte hypertrophy,24 supporting that elastin production is impaired in obesity and that it may have a role in glucose metabolism. The significance of the Exendin-4 induced decrease of elastin expression and secretion on AT function needs yet to be further clarified. Mechanical properties of ECM result from a balance between relative abundance of elastin and collagen, their efficient cross-linking by LOX and LOXL2 and their degradation, which is influenced by metalloproteinases and their inhibitors. Apart from the change in elastin, we did not observe any modification of LOX, LOXL2 or MMPs expression in response to Exendin-4 treatment, and cannot conclude an effect of GLP-1 to histological changes or mechanical properties of AT’s ECM, as technically not feasible. Similarly, the expression of TGFB1 and its downstream target CTGF, two key pro-fibrotic factors were not affected by Exendin-4, suggesting that GLP-1 does not induce a pro-fibrotic phenotype, though a change in the mechanical property by changing the ratio of collagen/elastin3 cannot be excluded.
GLP-1 and inflammation
Chronic low-grade inflammation and macrophage infiltration in AT have been reported to be closely related to the development of obesity and insulin resistance.25 We analysed the expression of inflammation markers in response to Exendin-4 treatment and found no change in either abdominal depot. Most importantly, we did not find changes in MCP-1 expression or secretion, whereas some studies suggest that 12 weeks treatment of obese patients with type-2 diabetes with the GLP-1R agonists, Exenatide decreases circulating cytokines including MCP-1 plasma concentrations independent of weight change, however, with a significant improvement in glycaemia.26 Other reports show that GLP-1 analogues inhibit expression of inflammatory cytokines in 3T3-L1 adipocytes.21 Nevertheless, anti-inflammatory properties of GLP-1 analogues have not been confirmed as direct effect and specific to human AT and several groups have seen no modification in MCP-1 or other cytokines’ plasma concentrations (which are likely to be attributable to AT inflammation) in non-obese subjects with type-2 diabetes despite weight loss,18 nor a change in AT expression of TNFα or MCP-1 in non-diabetic rats treated with Exenatide.27 An obvious direct effect of GLP-1 on AT inflammation independent of weight loss effects thus remains questionable. Clinical studies controlling for the improvement in glycaemia and the effect of weight loss, for example, by comparing patients with dietary induced weight loss of similar magnitude to the GLP-1 induced weight loss are needed.
Differential AT depot response
We found only OMAT responsive to Exendin-4 treatment. OMAT and abdominal SCAT exhibit different patterns of gene expression,28 OMAT has higher lipolytic activity15 and has as part of visceral AT a clear link with the development of metabolic syndrome characteristics (including glucose intolerance, hyperinsulinemia and hypertriglyceridemia) and cardiovascular disease.15 We postulated that the difference between OMAT and SCAT response could have been due to a differential expression of GLP-1R. We found more GPL1-R protein in SCAT, demonstrating that the different response to Exendin-4 is unlikely explained by the amount of GLP-1R. Higher expression of GLP-1R was found in OMAT of obese insulin-resistant subjects when compared with subjects with low insulin resistance,6 however, we were not able to reproduce these findings in morbidly obese subjects of whom most had diabetes. GLP-1R resistance in combination with insulin resistance cannot be excluded.
We acknowledge some study limitations. We may have missed peaks in mRNA expression occurring prior to 45 h of tissue culture and/or later mRNA changes. The duration was in part chosen to enable the study of cytokine and protein accumulation in the media. Similar to other in vitro studies,6, 7 we used 1–100 nM of Exendin-4 in our experiments. Even if previous pharmacokinetic studies showed that Exendin-4 treatment with current dosing results in circulating levels between 2.5 and 5 nM,29 higher doses of GLP-1 analogues are now under consideration for weight management.2 AT samples were used from non-diabetic overweight/obese subjects who are typically insulin resistant, whereas GLP-1 treatment is primarily for subjects with type-2 diabetes. We have expected an improvement in AT inflammation as reported in rodents and humans (circulatory/plasma inflammatory markers),26, 30 which we were unable to confirm and did not expect the need to stimulate inflammation in this in vitro explant model. Further studies are necessary, which include a model to take account of macrophage infiltration and polarisation within explant culture. We used normoglycaemic conditions as will be achieved by effective treatment of diabetes with GLP-1 analogues or when used in non-diabetic subjects for weight management. Furthermore, we were only able to study acute effects and can only hypothesise that they may become more pronounced with prolonged treatment.
In summary, Exendin-4 can directly modify expression of adiponectin and elastin in explant culture of human AT. Interestingly, OMAT, expansion of which is linked to the development of insulin resistance and the metabolic syndrome, seems to be more sensitive to Exendin-4, despite higher GLP-1 receptor expression in subcutaneous tissue. These results suggest that treatment of patients with type-2 diabetes and/or obesity with GLP-1 analogues could directly influence AT physiology, its properties and functions. However, apart from the positive effect on increased adiponectin expression/secretion we cannot verify an anti-inflammatory effect of GLP-1 on AT independent of weight loss.
References
Holst JJ . The physiology of glucagon-like peptide 1. Physiol Rev 2007; 87: 1409–1439.
Nuffer WA, Trujillo JM . Liraglutide: a new option for the treatment of obesity. Pharmacother J Hum Pharmacol Drug Ther 2015; 35: 926–934.
Alkhouli N, Mansfield J, Green E, Bell J, Knight B, Liversedge N et al. The mechanical properties of human adipose tissues and their relationships to the structure and composition of the extracellular matrix. Am J Physiol Endocrinol Metab 2013; 305: E1427–E1435.
Divoux A, Tordjman J, Lacasa D, Veyrie N, Hugol D, Aissat A et al. Fibrosis in human adipose tissue: composition, distribution, and link with lipid metabolism and fat. Diabetes 2010; 59: 2817–2825.
Clément K, Viguerie N, Poitou C, Carette C, Pelloux V, Curat CA et al. Weight loss regulates inflammation-related genes in white adipose tissue of obese subjects. FASEB J 2004; 18: 1657–1669.
Vendrell J, El Bekay R, Peral B, García-Fuentes E, Megia A, Macias-Gonzalez M et al. Study of the potential association of adipose tissue GLP-1 receptor with obesity and insulin resistance. Endocrinology 2011; 152: 4072–4079.
Robinson E, Cassidy RS, Tate M, Zhao Y, Lockhart S, Calderwood D et al. Exendin-4 protects against post-myocardial infarction remodelling via specific actions on inflammation and the extracellular matrix. Basic Res Cardiol 2015; 110: 20.
Goke R, Fehmann HC, Linn T, Schmidt H, Krause M, Eng J et al. Exendin-4 is a high potency agonist and truncated exendin-(9-39)-amide an antagonist at the glucagon-like peptide 1-(7-36)-amide receptor of insulin- secreting β-cells. J Biol Chem 1993; 268: 19650–19655.
McCulloch LJ, Rawling TJ, Sjöholm K, Franck N, Dankel SN, Price EJ et al. COL6A3 is regulated by leptin in human adipose tissue and reduced in obesity. Endocrinology 2015; 156: 134–146.
Kos K, Wong S, Tan B, Gummesson A, Jernas M, Franck N et al. Regulation of the fibrosis and angiogenesis promoter SPARC / osteonectin in human adipose tissue by weight. Diabetes 2009; 58: 1780–1788.
Kershaw EE, Flier JS . Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 2004; 89 (): 2548–2556.
Rodríguez A, Ezquerro S, Méndez-Giménez L, Becerril S, Frühbeck G . Revisiting the adipocyte: a model for integration of cytokine signaling in the regulation of energy metabolism. Am J Physiol Endocrinol Metab 2015; 309: E691–E714.
Kim YM, Kim EC, Kim Y . The human lysyl oxidase-like 2 protein functions as an amine oxidase toward collagen and elastin. Mol Biol Rep 2011; 38: 145–149.
Henegar C, Tordjman J, Achard V, Lacasa D, Cremer I, Guerre-Millo M et al. Adipose tissue transcriptomic signature highlights the pathological relevance of extracellular matrix in human obesity. Genome Biol 2008; 9: R14.
Wajchenberg BL . Subcutaneous and visceral adipose tissue: their relation to metabolic syndrome. Endocr Rev 2000; 21: 697–738.
Turer AT, Scherer PE . Adiponectin: mechanistic insights and clinical implications. Diabetologia 2012; 55: 2319–2326.
Drolet R, Bélanger C, Fortier M, Huot C, Mailloux J, Légaré D et al. Fat depot-specific impact of visceral obesity on adipocyte adiponectin release in women. Obesity (Silver Spring) 2009; 17: 424–430.
Bunck MC, Diamant M, Eliasson B, Cornér A, Shaginian RM, Heine RJ et al. Exenatide affects circulating cardiovascular risk biomarkers independently of changes in body composition. Diabetes Care 2010; 33: 1734–1737.
El Bekay R, Coín-Aragüez L, Fernández-García D, Oliva-Olivera W, Bernal-López R, Clemente-Postigo M et al. Effects of GLP-1 on the differentiation and metabolism of human adipocytes. Br J Pharmacol 2016; 173: 1820–1834.
Yang W-S, Lee W-J, Funahashi T, Tanaka S, Matsuzawa Y, Chao C-L et al. Weight reduction increases plasma levels of an adipose-derived anti inflammatory protein, adiponectin. J Clin Endocrinol Metab 2001; 86: 3815–3819.
Kim Chung LT, Hosaka T, Yoshida M, Harada N, Sakaue H, Sakai T et al. Exendin-4, a GLP-1 receptor agonist, directly induces adiponectin expression through protein kinase A pathway and prevents inflammatory adipokine expression. Biochem Biophys Res Commun 2009; 390: 613–618.
Shiraishi D, Fujiwara Y, Komohara Y, Mizuta H, Takeya M . Glucagon-like peptide-1 (GLP-1) induces M2 polarization of human macrophages via STAT3 activation. Biochem Biophys Res Commun 2012; 425: 304–308.
Spencer M, Unal R, Zhu B, Rasouli N, McGehee RE, Peterson CA et al. Adipose tissue extracellular matrix and vascular abnormalities in obesity and insulin resistance. J Clin Endocrinol Metab 2011; 96: E1990–E1998.
DeMarsilis AJ, Walji TA, Maedeker JA, Stoka KV, Kozel BA, Mecham RP et al. Elastin insufficiency predisposes mice to impaired glucose metabolism. J Mol Genet Med 2014; 8: 1–16.
Bastard J-P, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw 2006; 17: 4–12.
Chaudhuri A, Ghanim H, Vora M, Sia CL, Korzeniewski K, Dhindsa S et al. Exenatide exerts a potent antiinflammatory effect. J Clin Endocrinol Metab 2012; 97: 198–207.
Tanaka K, Masaki Y, Tanaka M, Miyazaki M, Enjoji M, Nakamuta M et al. Exenatide improves hepatic steatosis by enhancing lipid use in adipose tissue in nondiabetic rats. World J Gastroenterol 2014; 20: 2653–2663.
Vohl M, Sladek R, Robitaille J, Gurd S, Marceau P, Richard D et al. A survey of genes differentially expressed in subcutaneous and visceral adipose tissue in men. Obes Res 2004; 12: 1217–1222.
Ai G, Chen Z, Shan C, Che J, Hou Y, Cheng Y . Single- and multiple-dose pharmacokinetics of exendin-4 in rhesus monkeys. Int J Pharm 2008; 353: 56–64.
Lee YS, Park MS, Choung JS, Kim SS, Oh HH, Choi CS et al. Glucagon-like peptide-1 inhibits adipose tissue macrophage infiltration and inflammation in an obese mouse model of diabetes. Diabetologia 2012; 55: 2456–2468.
Acknowledgements
We acknowledge help of the staff from the Gynaecology department of the Centre for Women’s health and the RD&E NHS Foundation Trust. We thank all women who participated in this study. This programme was supported by the European Federation for the Study of Diabetes (EFSD/GSK 09).
Author contributions
EP, SJ, BK, NL and RW performed the experiments. EP, SJ, RW and KK analysed and interpreted the experimental results. EP and KK drafted the manuscript. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Nutrition & Diabetes website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Pastel, E., Joshi, S., Knight, B. et al. Effects of Exendin-4 on human adipose tissue inflammation and ECM remodelling. Nutr & Diabetes 6, e235 (2016). https://doi.org/10.1038/nutd.2016.44
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nutd.2016.44
This article is cited by
-
Role of adipose tissue GLP-1R expression in metabolic improvement after bariatric surgery in patients with type 2 diabetes
Scientific Reports (2019)
-
Regulation of visceral and epicardial adipose tissue for preventing cardiovascular injuries associated to obesity and diabetes
Cardiovascular Diabetology (2017)