Abstract
Background:
Sleep has been identified as having an influence on the success of weight-loss interventions; however, knowledge of the mechanisms and the extent to which sleep disturbances affect the magnitude of weight reduction is inconclusive.
Objective:
To determine if sleep duration and quality can predict the magnitude of weight reduction in a weight-loss intervention program for overweight and obese women.
Methods:
Ninety overweight and obese women aged 25–65 years completed the 7-month weight-loss phase of our weight-loss intervention. Sleep duration and quality were evaluated before the intervention by the Pittsburg Sleep Quality Index (PSQI), a self-report questionnaire, and by actigraphy. Serum levels of ghrelin, leptin, cortisol and insulin also were measured at baseline. Insulin resistance was measured by the homeostasis model assessment of insulin resistance (HOMA-IR).
Results:
The mean reduction rate of body mass index (BMI) after the intervention was 13.6%. Multiple linear regression revealed that the number of wake episodes (WEs) per night had a significant relationship with the reduction of BMI even after adjusting for other clinical variables (β=−0.341, P=0.001). The participants with five or more WEs per night (high-WE group) had a significantly lower reduction in BMI compared with those with fewer than five (normal-WE group), after adjusting for confounding variables. In contrast, the PSQI-assessed parameters, reflecting the subjective assessments of sleep quality and duration, failed to detect an association with the reduction in BMI. Baseline HOMA-IR was significantly higher in the high-WE group than in the normal-WE group after adjusting for confounding variables.
Conclusions:
Higher sleep fragmentation, as manifested by the increased number of WEs, predicts a lower magnitude of weight reduction in persons participating in weight-loss programs.
Similar content being viewed by others
Introduction
Obesity is a major healthcare problem that contributes to a number of diseases, including cardiovascular disease and diabetes mellitus, and it has enormous economic implications for society.1 The prevalence of obesity has increased markedly throughout the world in recent decades.2
The development and persistence of obesity are known to be influenced by a variety of environmental factors, such as excessive daily food intake and physical inactivity.2 Accumulating evidence also supports the hypothesis that sleep disturbance is an important contributing factor to obesity. Numerous epidemiological studies conducted in different geographical regions have investigated the association between sleep and obesity among different populations.3, 4, 5, 6, 7 The majority of these studies have found a relationship between short sleep duration and an increased risk of obesity.6 For example, a recent meta-analysis of 17 studies of 603 519 adults reported a pooled obesity odds ratio of 1.55 in those who had fewer than 5 hours of sleep, and a decrease in body mass index (BMI) by 0.35 kg m−2 for each additional hour of sleep.7
In addition to sleep duration, the quality of sleep has been suggested as an influential variable in the relationship between sleep and obesity.8, 9 In the multivariate analysis of the Cardiovascular Health Epidemiology Study,10 in which sleep duration and quality were assessed by the Pittsburg Sleep Quality Index (PSQI), the effect of sleep duration on the risk of obesity was rather modest. However, a significant association between poorer sleep quality and an increased BMI was found in women. The importance of sleep quality was reported in the Rotterdam Study,9 which found that BMI increased by 0.59 kg m−2 per s.d. of sleep fragmentation, as measured by actigraphy. This study also found that both short sleepers (<5 h or–6 h per night) and long sleepers (>8 h) were more likely to be obese in comparison with participants who slept between 7 and 8 hours, as measured by actigraphy. However, the association between short sleep and obesity was no longer significant after adjusting for actigraphy-assessed sleep fragmentation. Furthermore, self-reports of habitual sleep duration were not associated with BMI or obesity, which suggests the importance of conducting objective assessments in sleep studies.
It is clear that quantitative and qualitative aspects of sleep have profound effects on weight regulation. However, knowledge of how, and to what extent sleep disturbances affect weight regulation is inconclusive. There are a considerable number of discrepancies in study results based on subjective and objective parameters. To date, there are no studies that have reported the effects of objective sleep parameters on weight reduction in a weight-loss intervention program for obese people. If the effect of sleep on weight regulation can be measured before treatment, it should provide a useful tool for weight-loss intervention.
The aim of this study was to determine if objective and subjective sleep-related parameters at baseline predict the magnitude of weight loss in a sample of overweight and obese women, following a weight-loss intervention program.
Materials and Methods
Study design
This investigation was part of a randomized-controlled study consisting of a two-phase trial that examined two strategies for maintaining weight loss. The weight-loss phase provided an intensive program of cognitive behavioral therapy for weight loss that lasted 7 months. Participants who lost at least 5% of their initial body weight during the weight-loss phase were eligible for the 3-month program provided in the weight maintenance phase. In this phase, the participants were randomized to one of two weight-loss maintenance interventions, with follow-up through 34 months after their initial entry into the study. All of the participants provided informed consent, and the Institutional Review Boards of Kyushu University Hospital approved the study protocol.
Eligibility
All of the participants were women aged 20–65 years, with a BMI of 25 kg m−2 or higher. They were able to understand and complete self-report questionnaires written in Japanese without assistance, and had no physical impairment that would preclude simple exercise. Those who met the following criteria were excluded from the study: weight loss of >5 kg during the previous 6 months, current diagnosis of bulimia nervosa, past history of anorexia nervosa, current pregnancy or breast feeding, planning to become pregnant within the next 24 months, taking any form of medication that would affect body weight, suffering from any health disorder affecting body weight, receiving continuous positive airway pressure therapy for obstructive sleep apnea, receiving a current treatment for psychiatric disorders or planning to move within the next 10 months.
Recruitment and entry
We recruited participants through the local newspaper, the university website, posters in the university hospital or hospitals near the university and a television program. Those who were interested and potentially eligible were scheduled for an information session to learn more about the study. At the information session, the principal investigator provided the details of the study and answered questions from the participants. Those who met the eligibility criteria were enrolled in the study. After informed consent was obtained, we collected demographic, lifestyle and health history data on the participants.
Weight-loss intervention
Cooper et al.11 originally developed the cognitive behavioral therapy program for obese individuals. We modified it for use in a group-therapy program12, 13 that included the clinical guidelines on obesity of the US National Institutes of Health,14 Look AHEAD (Action for Health in Diabetes)15and the Diabetes Prevention Program as a framework.16 Our treatment program was conducted in small groups of ~10 individuals. It consisted of 35 group sessions, each of them lasing 90 min, and 5 individual sessions over the 44-week period. The sessions were conducted once a week for the first consecutive 34 sessions, and the remaining sessions were held once every 2 weeks.
Two doctors and two certified nutritionists were involved as the healthcare providers in the participants’ treatment. All participants were instructed to keep a daily food diary to track their consumption of all food and drinks, wear a pedometer and record their daily number of steps. They also were advised to reduce their dietary intake by 500 calories per day from their caloric intake at the beginning of their therapy. The nutritionists checked the nutritional balance of the participants’ diets by examining their recordings of their food intake, and advised participants about the importance of eating vegetables and reducing the consumption of fat foods and sweets. The participants were advised to increase their level of physical exercise to a moderate intensity, such as walking (8000–10 000 steps per day). Furthermore, a series of stress-management sessions also was included in the program.
Anthropometric and body composition measurements
Our staff measured the participants’ height, body weight, waist circumference and hip circumference. The participants were asked to weigh themselves once a week, on the morning of their session. The percent of body fat was measured at baseline and at the end of the weight loss phase using the Dual Energy X-ray Absorptiometry system (QDA4500A: HOLOGIC, Bedford, MA, USA).
Dietary intake and physical activity
From the diet and exercise logs that the participants recorded, we calculated the mean food calories and the number of steps that the participants took during the weight-loss phase.
Depression and anxiety
Depression was evaluated using the Japanese version of the Center for Epidemiologic Studies-Depression Scale (CES-D). The CES-D is a 20-item, self-report questionnaire. The scores range from 0–60, with a higher score indicating the presence of depressive symptoms.17 Anxiety was evaluated using the Japanese version of the State-Trait Anxiety Inventory (STAI). The STAI is a self-report questionnaire consisting of two scales, the state and trait forms (STAI-1 assesses state anxiety and STAI-2 assesses trait-anxiety). Each scale consists of 20 items that indicate the presence or absence of anxiety symptoms.18
Sleep parameters
To obtain objective sleep parameters, we used actigraphy (Micro-mini RC, Ambulatory Monitoring, Inc., Ardsley, NY, USA). The participants were instructed to wear an actigraph continuously over a period of 5–7 consecutive days and nights, on the non-dominant wrist upon their entry into the program. It was set for 1-min epochs, in zero-crossing mode. The data retrieved from the actigraph were downloaded to a personal computer using the automatic actigraphy interface unit. These data were analyzed and scored using Action W-2 version 2.4.20 software (Ambulatory Monitoring, Inc.), to calculate sleep quality parameters, including sleep latency (number of minutes before the start of the first 20-min block of sleep), sleep minutes (total minutes scored as sleep), sleep efficiency (100 × sleep minutes/duration from first 20-min block of sleep to the last one), wake episodes (WEs) (the number of continuous blocks of one-minute wake epochs), wake after sleep onset (total wake minutes during nocturnal sleep), mean WEs (mean duration of WEs), long WEs (the number of blocks of WEs lasting 5 min or more) and the longest WE (duration of the longest WE). As the average number of WE was reported to be 4.0 times per night in normal sleepers,19 we used 5 times per night as a reasonable cutoff point for the high-WE group. All actigraphy-based measures were averaged over the total number of nights.
Subjective sleep parameters also were evaluated by the Pittsburgh Sleep Quality Index (PSQI), a self-rated questionnaire that assesses sleep quality and disturbances over the preceding 1-month interval.20 The instrument consists of 19 items, plus a five-item rating provided by a bed partner who is not included in scoring. A total PSQI score is highly sensitive and specific in distinguishing good from poor sleepers,20 and has been validated in a number of populations.21 In the current study, sleep quality (total PSQI score), sleep duration and Question 10 b, from the apnea subscale, were used for statistical analyses.
Measurement of hormonal markers
Blood samples were drawn from a vein in the antecubital fossa in the morning (10:00–12:00 am). The separated serum samples were stored at −80 °C until they were analyzed. Serum hormone levels were measured using commercially available kits. Ghrelin, insulin and growth hormone were analyzed by enzyme-linked immunosorbent assay: (a) active-ghrelin, (Molecular Devices, Tokyo, Japan; intra-assay coefficient of variation (CV) 2.13% and interassay CV 8.10%); (b) insulin, (TOSHO, Tokyo, Japan; intra-assay CV 2.6% and interassay CV 2.8%); (c) growth hormone, (THOSHO; intra-assay CV 2.6% and interassay CV 2.8%). Serum leptin, insulin-like growth factor-1 and cortisol levels were analyzed by radioimmunoassay: (a) serum leptin, (Hitachi Aloka Medical, Ltd., Tokyo, Japan; intra-assay CV 5.3% and interassay CV 8.1% ); immunoradiometric: (b) insulin-like growth factor-1, (Hitachi Aloka Medical, Ltd; intra-assay CV 1.1% and interassay CV 2.2%) and electrochemiluminescent: (c) cortisol, (Roche Diagnostics, Tokyo, Japan; intra-assay CV 1.68% and interassay CV 2.12%). Insulin resistance was calculated according to the following formula: homeostasis model assessment-insulin resistance=fasting insulin (μU ml−1) × fasting glucose (mg dl−1)/405.
Statistical analysis
The changes in clinical data between baseline measures and those taken at the end of the weight-loss phase were calculated using the paired-t test. Pearson's correlation coefficient was performed to examine the associations among the reduction in BMI, demographic, clinical, psychological and sleep-related variables. Simple and multiple regression analyses were used to clarify which variable at baseline was a predictor of the reduction in BMI. Student's t-test and analysis of covariance were used to analyze the reduction in BMI and hormonal markers between the participants with five or more WEs per night and those below five. Dummy codes were applied to marital status (single=0, marriage=1), working status (working=0, not working=1) and smoking status (nonsmoker=0, smoker=1), in Pearson's correlation test, simple and multiple regression analyses and analysis of covariance. All of the continuous variables are expressed as the means±s.d. All statistical analyses were performed with the SPSS software package (version 19.0 J for Windows). A value of P<0.05 was considered significant.
Results
Recruitment and baseline characteristics
A total of 230 women were screened for inclusion in the weight-loss program and 119 were enrolled (Figure 1). In total, 90 of 119 participants (75.6%) completed the weight-loss phase of the program. Their baseline characteristics are presented in Table 1. The participants’ mean age and BMIs were 47.9 (25–65) years and 31.1 (25.1–46.0) kg m−2, respectively.
Flow diagram of participants. Of 230 persons who contacted us by telephone or e-mail to participate in the study, 81 were excluded because of not meeting inclusion criteria (n=45), schedule conflicts (n=20) or not being interested in the study (n=16). Of the remaining 149 persons, 14 did not appear in the group information session because of unknown reasons, and 6 refused to participate in the study after the group information session. Of the remaining 129 persons who attended the screening visit, 10 were excluded because of not meeting the inclusion criteria. The remaining 119 participants agreed to participate in the weight loss program. After the program was initiated, 29 persons dropped out because of their unwillingness to continue with the program (n=14), schedule conflicts (n=6), family problems (n=5) and health problems unrelated to the program (n=4). Complete sets of data were collected from 90 of the initial 119 participants.
Kinetics of body weight
Changes in the clinical data between the baseline measures and those taken at the end of the weight-loss phase are presented in Table 1. The mean number of kg lost and the percentage of BMI reduced were 10.3±4.8 kg and 13.6±0.1%, respectively. All participants who completed the weight-loss phase lost >5% of their initial body weight. In total, 65 of 90 (72%) participants lost 10% or more of their initial body weight.
Relationship between the reduction in BMI and the objective sleep parameters
The baseline averages of the participants’ sleep parameters as measured by actigraphy were as follows: sleep minutes=332 min, sleep efficiency=93%, and WEs=5.72 per night (Supplementary Table 1). A significant correlation was found between the reduction in BMI and sleep parameters (baseline sleep efficiency and WEs) (Table 2, Supplementary Figure 1). Sleep efficiency and WEs also correlated significantly with the reduction in body fat ratio (sleep efficiency: r=0.258, P=0.015 and WEs: r=0.239, P=0.03). However, the correlation between sleep efficiency and WEs with the reduced BMI was stronger than with the reduced body fat ratio; therefore, BMI was chosen as a dependent variable in the regression analysis.
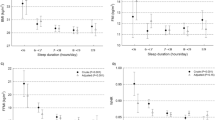
Simple regression analysis revealed that the reduction in BMI was associated with smoking status, the total calorie intake per day during treatment, and the number of WEs at baseline (Table 3). However, age, BMI, marital and working status, the CES-D, baseline STAI scores and the number of steps walked per day during treatment, did not reveal a significant correlation with the reduction in BMI. Because WEs had a high correlation with sleep efficiency and a higher correlation with BMI reduction than sleep efficiency (Table 2), only the number of WEs was entered as the independent variable in the multiple regression analysis to avoid multicollinearity, as shown in Table 3. The number of WEs remained significant even after adjusting for age, baseline BMI, smoking status, CES-D score and the total calorie intake per day during treatment. Of all the independent variables, the number of WEs also was the strongest predictor of BMI reduction (β=−0.341, P=0.002). As Figure 2 illustrates, the reduction in BMI was significantly different between the participants with fewer than five WEs per night (the normal-WE group) and those with five or more WEs per night (the high-WE group). This difference was found after adjusting for age, baseline BMI, smoking status, CES-D score, average daily caloric intake and average number of daily steps during treatment, using analysis of covariance (F=6.289, P=0.014).
Differences in BMI reduction between the normal- and high-WE groups. All data are expressed as the mean±s.d. The mean reduction of BMI in the normal- and high-WE group was 15.4% and 11.7%, respectively. The difference was significant after adjusting for age, baseline BMI, smoking status, CES-D scores, average daily caloric intake and average daily number of steps during treatment using ANCOVA (F=6.289, *P=0.014).
Among the subjective measures of sleep assessment, sleep duration as measured by the PSQI, exhibited a linear relationship with sleep minutes, as measured by actigraphy (r=0.229, P=0.0127). The PSQI total score was not associated with any of the acitigraphy-measured sleep parameters. The apnea subscale of the PSQI correlated only with wake after sleep onset, as assessed by actigraphy (r=0.250, P=0.0326). However, none of the three PSQI parameters (sleep duration, sleep quality and apnea) were correlated with BMI reduction (sleep duration: r=−0.178, P=0.186; sleep quality: r=−0.0127, P=0.905; and apnea: r=−0.0363, P=0.760).
Relationship between hormonal markers and WEs
Analysis of covariance on the baseline hormonal markers showed that homeostasis model assessment of insulin resistance and cortisol levels were significantly different between the normal- and the high-WE groups. None of the other baseline hormonal markers differed between the two groups (Table 4).
Discussion
In the current study, highly fragmented sleep, as revealed by the increased number of WEs on an actigraph, predicted a lower magnitude of weight loss in a sample of overweight and obese women participating in a weight-loss intervention. These results support the current view that sleep quality is an important factor contributing to weight reduction and indicate the importance of using objective sleep measures in the assessment of sleep quality.
Several clinical studies with longitudinal designs have found that baseline parameters of sleep can predict the efficacy of a long-term weight-loss intervention program. For example, sleep duration was reported to predict success in an intensive 6-month behavioral weight-loss program in 472 obese adult participants.22 Thomson et al.23 examined the baseline measures of sleep duration and quality of 245 overweight or obese women who participated in a 24-month commercial multifaceted weight-loss program, and thereafter, evaluated how sleep parameters assessed by the PSQI affected weight reduction. They found that in addition to the participants who reported sleeping ⩽7 h per night, those with poor subjective sleep quality were significantly less likely to achieve successful weight loss. These findings suggest that sleep quality and sleep duration can predict the efficacy of long-term weight-loss interventions. However, because some of the sleep parameters were measured by subjective instruments (that is, self-report questionnaires), these sleep measures may be biased by the participants’ own perceptions. The current study used actigraphy to assess the effects of sleep quality on weight loss, and the results suggested the importance of using objective sleep measures in addition to subjective ones in the assessment of sleep quality.
In contrast to the studies mentioned above, our findings revealed that sleep duration at baseline was not associated with the degree of weight loss, as expected. The reason for this discrepancy is unclear so far. However, it might be related to a difference in the methods used to assess sleep parameters, because the other studies assessed sleep duration and quality using only self-report questionnaires.22, 23 Nevertheless, we cannot rule out the importance of sleep duration when considering the risk or prevention of vulnerability to obesity. Further studies are needed to clarify whether short sleep duration per se or short sleep due to frequent interruptions causes an increased risk of obesity.
In this study, WEs, which were strongly related to sleep quality, significantly contributed to the magnitude of weight loss, even after adjusting for confounding factors, such as the number of steps walked and total calorie intake, which are known to be key factors in weight-loss success. The precise mechanism, which explains the effect of fragmented sleep on weight loss, remains to be elucidated; however, several possible pathways or mechanisms have been suggested. Stamatakiset al.24 elicited fragmented sleep across all of the sleep stages in 11 healthy volunteers for two nights in a laboratory setting, using auditory and mechanical stimuli. They showed that sleep fragmentation decreased both insulin sensitivity and glucose effectiveness. Similarly, in a 24 h randomized crossover study, it was reported that a single night of fragmented sleep resulted in reduced REM sleep, a shift in insulin concentration and a decrease in glucagon-like peptide-1 concentrations.25 These findings are consistent with the results of the current study showing that homeostasis model assessment of insulin resistance was significantly higher in the high-WE group compared with the normal-WE group, suggesting that fragmented sleep may attenuate the magnitude of weight loss by inducing insulin resistance in the hosts.25
In the current study, serum cortisol levels were lower in the high-WE group than in the normal group, indicating that sleep fragmentation may cause dysregulation of the hypothalamic-pituitary-adrenal axis. Previous studies have found that nocturnal awakenings are associated with an evening pulsatile release of cortisol, which is followed by a temporary inhibition of cortisol secretion the following morning.26, 27 These findings suggest that wakefulness may increase negative feedback sensitivity of the hypothalamic-pituitary-adrenal axis.28 Karaca et al.29 suggested that the enhanced sensitivity of the hypothalamic-pituitary-adrenal axis to the negative feedback effect of cortisol may lead to decreased cortisol levels in the morning. Although we did not perform real-time monitoring of serum cortisol levels in this study, the decreased level of cortisol in the morning in the high-WE group might accompanied the elevated levels of evening cortisol, similar to the findings of previous studies.26, 27 Furthermore, increased cortisol levels in the evening have been reported to be associated with decreased insulin sensitivity the following morning.30, 31 This may lead to the development of insulin resistance over time.32 Taken together, increased levels of nocturnal cortisol induced by sleep fragmentation may explain the increased insulin resistance in the high-WE group and result in difficulties with weight reduction in diet interventions.
We failed to find associations between appetite-related hormones, such as leptin and ghrelin, and WEs, although a previous study suggested a possible relationship between plasma leptin levels and fragmented sleep. In an animal experiment, fragmented sleep induced an increase in weight gain and changed plasma leptin levels, implicating the possible involvement of plasma leptin in weight gain.33 Further studies are needed to clarify the role of appetite-related hormones in the relationship between sleep disturbances and weight regulation in humans.
It should be noted that the present study has several limitations. First, our sample size is relatively small and we cannot apply our results to obese men because all of the participants were women. Second, we cannot exclude the possibility that variables other than those we examined, such as sleep apnea and environmental noise, may have affected our results. Third, the dropout rate (24.3%) of the participants was relatively high, which might have contributed to a selection bias in the results. Finally, although the association between fragmented sleep and weight loss was an important observation, further intervention studies should be conducted to demonstrate the causality of this observation.
In summary, baseline measures of sleep fragmentation may predict the magnitude of weight reduction in weight-loss interventions. Clearly, further studies are needed to clarify whether or not improving sleep quality may actually increase the body’s efficiency in weight reduction. Such studies could facilitate the development of novel strategies to tackle the pervasive problem of obesity.
References
Withrow D, Alter DA . The economic burden of obesity worldwide: a systematic review of the direct costs of obesity. Obes Rev 2011; 12: 131–141.
Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014; 384: 766–781 (in press).
Itani O, Kaneita Y, Murata A, Yokoyama E, Ohida T . Association of onset of obesity with sleep duration and shift work among Japanese adults. Sleep Med 2011; 12: 341–345.
Magee CA, Iverson DC, Caputi P . Sleep duration and obesity in middle-aged Australian adults. Obesity (Silver Spring) 2010; 18: 420–421.
Patterson RE, Emond JA, Natarajan L, Wesseling-Perry K, Kolonel LN, Jardack P et al. Short sleep duration is associated with higher energy intake and expenditure among African-American and non-Hispanic white adults. J Nutr 2014; 144: 461–466.
Beccuti G, Pannain S . Sleep and obesity. Curr Opin Clin Nutr Metab Care 2011; 14: 402–412.
Cappuccio FP, Taggart FM, Kandala NB, Currie A, Peile E, Stranges S et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep 2008; 31: 619–626.
Lauderdale DS, Knutson KL, Rathouz PJ, Yan LL, Hulley SB, Liu K . Cross-sectional and longitudinal associations between objectively measured sleep duration and body mass index: the CARDIA Sleep Study. Am J Epidemiol 2009; 170: 805–813.
van den Berg JF, Knvistingh Neven A, Tulen JH, Hofman A, Witteman JC, Miedema HM et al. Actigraphic sleep duration and fragmentation are related to obesity in the elderly: the Rotterdam Study. Int J Obes (Lond) 2008; 32: 1083–1090.
Bidulescu A, Din-Dzietham R, Coverson DL, Chen Z, Meng YX, Buxbaum SG et al. Interaction of sleep quality and psychosocial stress on obesity in African Americans: the Cardiovascular Health Epidemiology Study (CHES). BMC Public Health 2010; 10: 581.
Cooper Z, Fairburn C, Hawker D . Cognitive–behavioral treatment of obesity; a clinician’s Guide 2003. Guilford Press: New York, NY, USA, 2003.
Nozaki T, Sawamoto R, Sudo N . Cognitive behavioral therapy for obesity. Nihon Rinsho 2013; 71: 329–334 (Japanese).
Nozaki T, Sawamoto R, Furukawa T, Tnahashi T, Morita C, Hata T et al. Group based cognitive behavioral therapy for obesity. Jpn J Psychosm Int Med 2013; 17: 220–225 (Japanese).
Anonymous Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults--The Evidence Report. National Institutes of Health. Obes Res 1998; 6 ((Suppl 2)): 51S–209S.
Look AHEAD Research Group, Look AHEAD Research Group, Wing RR . Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four-year results of the Look AHEAD trial. Arch Intern Med 2010; 170: 1566–1575.
Diabetes Prevention Program (DPP) Research Group. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care 2002; 25: 2165–2171.
Radloff L . The CES-D Scale: a Self-Report Depression Scale for Research in the General Population. Appl Psychol Measur 1997; 1: 385–401.
Spielberger CR, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA . Manual for the state-trait anxiety inventory (STAI). Consulting Psychologists Press: Palo Alto, CA, USA, 1983.
Tajima S, Kuratsune H, Yamaguti K, Takahashi A, Takashima S, Watanabe Y et al. Estimation of fatigue state in patient with CFS using actigraph and R-R interval power spectrum analysis. Nihon Rinsho 2007; 65: 1057–1064 (Japanese).
Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ . The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989; 28: 193–213.
Carpenter JS, Andrykowski MA . Psychometric evaluation of the Pittsburgh Sleep Quality Index. J Psychosom Res 1998; 45: 5–13.
Elder CR, Gullion CM, Funk KL, Debar LL, Lindberg NM, Stevens VJ . Impact of sleep, screen time, depression and stress on weight change in the intensive weight loss phase of the LIFE study. Int J Obes (Lond) 2012; 36: 86–92.
Thomson CA, Morrow KL, Flatt SW, Wertheim BC, Perfect MM, Ravia JJ et al. Relationship between sleep quality and quantity and weight loss in women participating in a weight-loss intervention trial. Obesity (Silver Spring) 2012; 20: 1419–1425.
Stamatakis KA, Punjabi NM . Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest 2010; 137: 95–101.
Gonnissen HK, Hursel R, Rutters F, Martens EA, Westerterp-Plantenga MS . Effects of sleep fragmentation on appetite and related hormone concentrations over 24 h in healthy men. Br J Nutr 2013; 109: 748–756.
Follenius M, Brandenberger G, Bandesapt JJ, Libert JP, Ehrhart J . Nocturnal cortisol release in relation to sleep structure. Sleep 1992; 15: 21–27.
Ekstedt M, Akerstedt T, Soderstrom M . Microarousals during sleep are associated with increased levels of lipids, cortisol, and blood pressure. Psychosom Med 2004; 66: 925–931.
Spath-Schwalbe E, Gofferje M, Kern W, Born J, Fehm HL . Sleep disruption alters nocturnal ACTH and cortisol secretory patterns. Biol Psychiatry 1991; 29: 575–584.
Karaca Z, Ismailogullari S, Korkmaz S, Cakir I, Aksu M, Baydemir R et al. Obstructive sleep apnoea syndrome is associated with relative hypocortisolemia and decreased hypothalamo-pituitary-adrenal axis response to 1 and 250mug ACTH and glucagon stimulation tests. Sleep Med 2013; 14: 160–164.
Plat L, Leproult R, L'Hermite-Baleriaux M, Fery F, Mockel J, Polonsky KS et al. Metabolic effects of short-term elevations of plasma cortisol are more pronounced in the evening than in the morning. J Clin Endocrinol Metab 1999; 84: 3082–3092.
Van Cauter E, Polonsky KS, Scheen AJ . Roles of circadian rhythmicity and sleep in human glucose regulation. Endocr Rev 1997; 18: 716–738.
McNeil J, Doucet E, Chaput JP . Inadequate sleep as a contributor to obesity and type 2 diabetes. Can J Diabetes 2013; 37: 103–108.
Wang Y, Carreras A, Lee S, Hakim F, Zhang SX, Nair D et al. Chronic sleep fragmentation promotes obesity in young adult mice. Obesity (Silver Spring) 2014; 22: 758–762.
Acknowledgements
This study was supported by a Research Grant (23-3) for Nervous and Mental Disorders from the Ministry of Health, Labour and Welfare of Japan, a Grant-in-Aid for Scientific Research (25460902) from the Japan Society for the Promotion of Science, and a Budget for clinical research base development project(GAQQ250001) from the Ministry of Health, Labour and Welfare of Japan. The authors thank Junko Sakaguchi and Akemi Kugimaru, national registered nutritionists, for nutritional guidance. We also thank Junji Kishimoto, PhD, for his contribution to the statistical analysis, and Ayako Tomihisa and Miyuki Ito for their assistance with the collection and recording of data in the registers.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Nutrition & Diabetes website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Sawamoto, R., Nozaki, T., Furukawa, T. et al. Higher sleep fragmentation predicts a lower magnitude of weight loss in overweight and obese women participating in a weight-loss intervention. Nutr & Diabetes 4, e144 (2014). https://doi.org/10.1038/nutd.2014.41
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nutd.2014.41
This article is cited by
-
Effect of sleep on weight loss and adherence to diet and physical activity recommendations during an 18-month behavioral weight loss intervention
International Journal of Obesity (2022)
-
Sleep characteristics modify the associations of physical activity during pregnancy and gestational weight gain
Archives of Gynecology and Obstetrics (2022)
-
The association between sleep health and weight change during a 12-month behavioral weight loss intervention
International Journal of Obesity (2021)
-
Sleep Extension: A Potential Target for Obesity Treatment
Current Diabetes Reports (2020)
-
Sleep, quality of life, and depression in endometrial cancer survivors with obesity seeking weight loss
Supportive Care in Cancer (2020)