Abstract
Objective:
To compare the effects of weight loss on visceral and subcutaneous abdominal fat, liver and pancreas lipid content and to test the effects of these changes on metabolic improvement observed after weight loss.
Design:
Weight-loss program designed to achieve a loss of 7–10% of the initial weight.
Subjects:
24 obese subjects (13 males and 11 females) with age ranging from 26 to 69 years and body mass index (BMI) 30.2–50.5 kg m−2. Measurements: weight, BMI, waist circumference, body composition as assessed by dual-energy X-ray absorptiometry, metabolic variables, leptin, adiponectin, visceral and subcutaneous abdominal fat, liver and pancreas lipid content as assessed by magnetic resonance were evaluated before and after weight loss achieved by hypocaloric diet.
Results:
After a mean body weight decrease of 8.9%, BMI, waist circumference, fat mass, all metabolic variables, homeostasis model assessment of insulin resistance (HOMA), alanine amino transferase, gamma glutamyl transpeptidase, high-sensitivity C-reactive protein (hs-CRP) and leptin, but not adiponectin and high-density lipoprotein-cholesterol, significantly decreased (all P<0.01). Visceral and subcutaneos abdominal fat, liver and pancreas lipid content significantly decreased (all P<0.01). Percent changes in liver lipid content were greater (84.1±3%) than those in lipid pancreas content (42.3±29%) and visceral abdominal fat (31.9±15.6%). After weight loss, percentage of subjects with liver steatosis decreased from 75 to 12.5%. Insulin resistance improvement was predicted by changes in liver lipid content independently of changes in visceral fat, pancreas lipid content, systemic inflammation, leptin and gender.
Conclusion:
Moderate weight loss determines significant decline in visceral abdominal fat, lipid content in liver and pancreas. Reduction of liver lipid content was greater than that of pancreas lipid content and visceral fat loss. Liver lipid content is the strongest predictor of insulin resistance improvement after weight loss.
Similar content being viewed by others
Introduction
Several studies showed that even moderate weight loss improves metabolic complications of obesity.1, 2 This may be due to a preferential loss of visceral fat compared with subcutaneous3, 4 or may also be due to a reduction in the ectopic fat content.5, 6, 7, 8, 9 In fact, it has been demonstrated that ectopic fat deposition (i.e., liver, pancreas and muscle fat infiltration) significantly contributes to insulin resistance and metabolic alterations observed in obese subjects.6, 7 Recently, in a murine model it has been shown that impairment of glucose homeostasis and insulin resistance are associated with hepatosteatosis9 and triglyceride overaccumulation in the pancreas,10 consequent to long-term high-fat feeding. Using computed tomography at the midthigh level, a significant decrease in muscle lipid content after moderate weight loss has been observed in obese sedentary subjects.5, 6, 8
Using magnetic resonance, Colles et al.11 observed a significant decline in liver volume, a proxy of liver fat infiltration, after a mean weight loss of 10% in obese subjects. In eight obese subjects with type 2 diabetes, Peterson et al.12 observed that a weight loss of 8 kg normalized fasting glucose and that this improvement was associated with an 81% decline of intrahepatic lipid content.
To the best of our knowledge, no studies have evaluated the effect of weight loss on pancreas lipid content in obese people or the possible contribution of its decrease to the metabolic improvement observed after weight loss. Furthermore, no studies compared the relative simultaneous decline in liver and pancreas ectopic fat deposition after weight loss.
The aim of this study was to compare the effect of weight loss on visceral and subcutaneous abdominal fat and on liver and pancreas fat content. A further aim was to test the combined and separate effects of these simultaneous declines on metabolic improvement. Magnetic resonance, considered the gold standard for assessment of body composition,13 was used to quantify visceral and subcutaneous adipose tissue as well as liver and pancreas lipid content before and after weight loss.
Materials and methods
Subjects
A total of 24 subjects (13 men and 11 women) with mean age 46.7±14.3 years and mean body mass index (BMI) 35.4±4.5 kg m−2, were included in the study. All subjects were in good general health, as determined by a complete medical history and physical examination, as well as a normal blood count, chemical screening battery and urine analysis except for obesity, hypercholesterolemia (42.8%), hypertension (35.7%). No patients had previously diagnosed diabetes according to American Diabetes Association,14 21.4% were smokers. Subjects with fasting plasma glucose ⩾7 mM l−1 (two men and one woman) were excluded from the study as well as subjects with daily alcohol consumption, >30 g for men and >20 g for women.15 All women were post-menopausal and not on hormonal replacement therapy. None of the participants was regularly engaged in physical activity or changed his physical activity level during the study. All participants gave their informed consent and the experimental protocol was approved by the Ethical Committee of our University.
Anthropometric measurements
With the subjects wearing light indoor clothes and no shoes, body weight was measured to the nearest 0.1 kg. (Salus scale, Milan, Italy), and height to the nearest 0.5 cm using a stadiometer (Salus stadiometer). BMI was calculated as body weight adjusted by stature squared (kg m−2). Waist circumference was obtained with a measuring tape as the minimum circumference between the xyphoid process and the umbilicus.
Body composition assessment
Body composition was measured using dual-energy X-ray absorptiometry (Hologic QDR 4500, Waltham, MA, USA) array beam System Software Version 11.1.
The characteristics and physical concepts of dual-energy X-ray absorptiometry measurement have been described elsewhere.16 Daily quality-assurance tests were performed according to the manufacturer's directions. All scans were subsequently analyzed by a single trained investigator. Total body fat was expressed in kg (fat mass) and as a percentage of body weight (fat mass %).
Magnetic resonance data acquisition
MRI (magnetic resonance imaging) was performed with a 1.5 T magnet (Symphony, Siemens Medical Systems, Erlangen, Germany) and two body phased-array coils. The imaging protocol included an axial T1-weighted dual-phase gradient-echo sequence (repetition time ms/echo time ms, 96/2.33 (opposed phase), 96/4.85 (in phase); flip angle 70°; matrix 320 × 320; field of view 500 cm) of the upper abdomen and an axial T1-weighted gradient-echo sequence (TR 90; TE 3.46; flip angle 70°; matrix 256 × 256; field of view 500 cm) from L3 to L5. APR and GAZ rated the quality of all MR images on a scale of 1 to 5 (5 being optimal signal to noise ratio, 1 being unreadable) as previously described.17 The average MR quality was 4.1 (ranging from 3 to 5).
Abdominal fat evaluation
All images were analyzed using Sliceomatic image analysis software (version 4.2, Tomovision, Montreal, Canada) and a single reader measured visceral adipose tissue (AT) and subcutaneous AT on a single slice at L4–L5 for men and women, as previously described.13
Abdominal subcutaneous AT area was defined as the area of adipose tissue between the skin and the outermost aspect of the abdominal muscle wall. All the adipose tissue pixels within the abdominal cavity between the innermost aspect of the abdominal and oblique wall musculature and the anterior aspect of the vertebral body were considered visceral abdominal AT. Interclass correlations for intra-reader comparisons in a subgroup of 20 subjects were 0.996 for subcutaneous AT, 0.993 for visceral AT.
Ectopic fat infiltration evaluation
MR images were reviewed on a commercially available workstation (Leonardo, Siemens Medical Systems) by a single reader who was unaware of laboratory and clinical results.
The technique used to measure SI (signal intensity) values from regions of interest (ROIs) in the liver and spleen and to calculate the relative SI losses of the liver and the other organs was based on methods previously described for the liver.18 The percentage of relative SI loss on opposed-phase MR images was considered to be a reasonable measurement of liver fat on the basis of the known effect of fat on SI values. The SI values of the liver and spleen were recorded on in- and opposed-phase T1-weighted MR images by positioning circular ROIs at anatomically matched locations on paired sequences, avoiding visible vessels, abnormalities and artefacts. Three circular ROIs were positioned in the liver (left lobe, upper right lobe and lower right lobe), three in the pancreas (head, body and tail, avoiding the main pancreatic duct), one each in the psoas muscles. The s.d. of the SI measurements within each ROI was kept to less than 10%. The SI of the spleen was similarly measured by positioning two circular ROIs in the splenic parenchyma. Size of the ROI was variable in the different organs: 1–2 cm diameter for the liver and spleen, equal or smaller for the pancreas. When more than one ROI was positioned in an organ, a mean SI was calculated to account for signal heterogeneity.
Liver fat was estimated on opposed-phase MR images as the percentage of relative SI loss of the liver on opposed-phase images with a previously used formula18

where Lin is in-phase mean liver SI, Sin is mean in-phase spleen SI, Lop is mean opposed-phase liver SI and Sop is mean opposed-phase spleen SI.
Similar formulas were used to calculate the amount of fat in the pancreas according to previous reports.17, 19, 20 The grading system for liver steatosis was based on that used in earlier studies:21, 22 grade 0 corresponding to less than 5% steatosis; grade 1 to 6–33% steatosis; grade 2 to 34–66% steatosis; and grade 3 to greater than 66% steatosis. The grading system incorporates the accepted normal value of histopathological liver fat, which is less than 5%, and is the standard applied in the clinical assessment of severity of liver steatosis. We calculated the intra- and inter-observer variability of our MRI measurements for pancreatic fat content and liver fat content, which were 8.2% and 9.7% and 7.8% and 8.2%, respectively (as determined in a subset of 20 obese patients).17
Biochemical analyses
Venous blood samples for all metabolic assessments were obtained after overnight fast. Plasma glucose was measured using a glucose analyzer (Beckman Instruments Inc., Palo Alto, CA, USA). The intra-assay coefficient of variation was 1.5%.
Plasma immune-reactive insulin underwent duplicate measurements by double-antibody radioimmunoassay using a commercial kit (Diagnostic Products Corp., Los Angeles, CA, USA). Sensitivity was 6 pM l−1 and the intra-assay coefficient of variation was 4.9%.
Insulin resistance was estimated with the HOMA (homeostasis model assessment of insulin resistance) method.23
Cholesterol and triglyceride levels were determined using a Technicon Auto analyzer (Technicon Inc., Co, Tarrytown, NY, USA) and dextran-magnesium precipitation was used to separate high-density lipoprotein.
Serum leptin was measured using a specific ELISA kit (DBC-Diagnostic Biochem Canada Inc., London, Canada). Sensitivity was 0.5 ng ml−1 and the intra-assay and interassay coefficient of variations were 7.4% and 9.6%, respectively.
Serum adiponectin was measured with a commercially available ELISA kit (B-Bridge International, Inc., Sunnyvale, CA, USA). Sensitivity was 20 pg ml−1 and the intra-assay and interassay coefficient of variations were 3.5% and 5.2%, respectively.
Dietary intake
A trained dietician performed a 7-day dietary recall interview in order to assess the dietary habits of each subject enrolled in the study. A recall grid representing 7 days of the prior week and all possible food-encounter times was used by the dietician. Portion sizes were estimated for foods and fluids by comparing with reference foods and fluids in a booklet of photographs. The interview takes approximately 40 min, on average, for the study subject to complete. The record data were then processed by the dietician using special software to calculate daily intake of energy, protein, fat, carbohydrate and alcohol based on the tables furnished by the Italian National Institute of Nutrition.24
Hypoenergetic diet
All the subjects completed a weight-loss program designed to achieve a loss of 7–10% of the initial weight. The caloric restriction was 500 kcal below the resting energy expenditure, as evaluated by indirect calorimetry and multiplied by a physical activity level of 1.4. Each subject received a diet providing 62% carbohydrates, 24% fat, 14% protein and 20 g fiber. The only beverage allowed was water. The subjects underwent monthly clinical and nutritional follow-ups. Dietary compliance was checked by a 24-h recall every 4 weeks during an outpatient visit.
Statistical analyses
Results are shown as means±s.d. Log transformations were performed for non-normal variables. Comparisons of anthropometric, metabolic and body-composition variables before and after weight loss were made by using paired t-test. Mcnemar test was used to test changes in the prevalence of liver steatosis before and after weight loss. Correlation analyses were used to test associations between variables. Linear regression analyses were used to test the joint effects of changes in liver and pancreas lipid content, as well as changes in leptin, high-sensitivity C-reactive protein (hs-CRP), visceral AT and waist circumference after weight loss on HOMA changes. The level of statistical significance was P<0.05 for all the variables. All statistical analyses were performed using the SPSS statistical package.25
Results
The flowchart of study participants is shown in Figure 1. Anthropometric, body composition, fat distribution, hs-CRP, adipokines and metabolic variables before and after weight loss are shown in Table 1. The mean decrease in body weight was 8.9%. BMI and waist circumference significantly decreased after weight loss (both P<0.001) as well as fat mass and fat mass % (both P<0.001). Visceral, subcutaneous and total abdominal AT significantly decreased (all P<0.001). Among the metabolic variables, glucose, insulin, HOMA, total cholesterol, triglycerides and leptin significantly decreased (all P<0.01), whereas adiponectin did not change. A significant decline was also observed in gamma glutamyl transpeptidase and alanine amino transferase (both P<0.001).
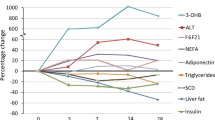
Changes in liver and pancreas lipid content in each of the subjects enrolled in the study expressed as mean±s.d. values are shown in Figures 2a and b. Liver lipid content significantly decreased, as well as pancreas lipid content (P<0.001 and P=0.001, respectively). Interestingly, percent changes in liver lipid content were greater (84.1±3%) than those in pancreas lipid content (42.3±29%), visceral abdominal AT (31.9±15.6%) and subcutaneous abdominal AT (13.6±25.9%).
As far as hepatic steatosis, as assessed by MRI, 75% (18/24) patients showed steatosis at baseline: respectively 50% (12/24) grade 1 steatosis, 12.5% (3/24) grade 2 steatosis and 12.5% (3/24) grade 3 steatosis. After weight loss, the percentage of subjects with steatosis significantly decreased to 12.5% (P=0.008) and none of these subjects had grade 2 or 3 steatosis (Figure 3).
Table 2 shows the correlations between changes in anthropometric variables, body composition, biochemical variables and changes in liver and pancreas lipid content and HOMA index after weight loss.
The loss of liver lipid content was related with the decrease in visceral AT (r=0.664, P<0.001), subcutaneous AT (r=0.505, P=0.012) and hs-CRP (r=0.554, P=0.005). Pancreas lipid infiltration decrease was related to visceral AT decrement after weight loss (P<0.001). An association between the respective changes in the liver and pancreas lipid content was also observed (P=0.033).
HOMA improvement was associated with reduction in waist circumference, visceral and subcutaneous abdominal AT and change in hs-CRP and insulin.
To evaluate independent determinants of insulin resistance improvement, a linear regression analysis was performed considering changes in HOMA index as dependent variable and the variables most closely correlated with it as independent variables. Table 3 shows different models considering gender, changes in liver and pancreas lipid content, visceral AT (or alternatively waist), leptin and hs-CRP as determinants of HOMA improvement.
The proportion of variance explained by gender and change in liver lipid content was 55%. When change in waist was added, 65.3% of variance was explained. When change in hs-CRP was also included in the model, a further increase in the proportion of variance was observed (71.9%) and F-test value decreased to 12.138. Building a model considering gender, changes in liver lipid content, visceral AT and hs-CRP as independent variables, 62.9% of variance in liver lipid content was explained.
A 49.7% and 49.1% of variance was explained, respectively, building models using gender and waist or alternatively gender and visceral AT.
Discussion
Our study shows that a moderate weight loss reduces visceral AT as well as hepatic and pancreatic lipid content. After weight loss, percent changes are greater in liver lipid content than in pancreas lipid content and visceral abdominal AT. Furthermore, insulin resistance, hs-CRP and leptin were significantly reduced after weight loss. Changes in liver lipid content were the best predictors of insulin resistance improvement after weight loss independently of changes in visceral fat distribution, pancreas lipid content, leptin, systemic inflammation as evaluated with hs-CRP and gender.
As expected, in agreement with previous studies,4, 26 after weight loss the amount of visceral abdominal AT loss was higher than that of subcutaneous abdominal AT. Preferential loss of visceral abdominal AT during initial modest weight loss, due to its higher lipolysis response, has been reported.26
After moderate weight loss, we also observed a significant loss in all ectopic fat compartments in our subjects. The decrease in liver lipid content after weight loss has been previously reported in patients with type 2 diabetes,12, 27 overweight28 and obese.29, 30
Interestingly, a decline in body fat % of about 3 units (from 36.4 to 33.5, Table 1), as in our study, has been shown to be associated with a much greater decline in liver lipid content and with a nearly total disappearance of liver steatosis. In fact, in our study sample, 75% of subjects had liver steatosis, as estimated according to Bahl et al.22 by liver SI losses higher than 3%, at the beginning of the study and only 12.5% still had after weight loss, thus suggesting that a moderate weight loss is a landmark treatment for this condition.
To the best of our knowledge, no studies have evaluated changes in pancreas lipid content after weight loss. Our study shows a more than 40% reduction of pancreas lipid content after a moderate weight loss. Pancreas lipid content raises great interest because it has been suggested that fat deposition in and around pancreatic islets could be associated with impaired beta cells function in humans.31, 32 However, its decrease after moderate weight loss, as reported in our subjects, could contribute to the improvement of insulin resistance following weight loss.
Our data support that even moderate weight loss may decrease ectopic fat deposition in both, liver and pancreas, and that the amount of the decline in each compartment may be different. In fact, in our study group, mean changes in liver lipid content were greater than those in pancreas lipid content as well as than in visceral abdominal AT. Our findings of greatest decline in liver lipid content are at least partially in line with those of Colles et al.,11 who demonstrated that intra-hepatic fat was mobilized faster than visceral and subcutaneous fat and with those of others.8, 12, 27, 28, 29 Actually, our results seem to complement and expand these previous findings by also giving information on the effects of weight loss on pancreas lipid content.
Excess liver and pancreas lipid content have been shown to be frequently present in the same patients: Lee et al.33 showed that concurrence of fatty pancreas and fatty liver, as estimated by sonography, was found in 70% of subjects and fatty liver without fatty pancreas just in 2%. However, our findings seem to suggest that dietary restriction may mobilize more ectopic fat stored in the liver than in the pancreas.
It has been recently observed, by using immuno-histochemistry, that fat in human pancreas could be stored in adipocytes between pancreatic cells in addition to vacuoles in pancreatic cells.34
No similar evidence has been shown in liver where fat is located inside hepatic cells; thus our findings of a higher decline of lipid content in liver than in pancreas could be at least partially explained by the fact that weight loss may easily mobilize triglycerides located inside pancreatic or hepatic cells than those located inside adipocytes between pancreatic cells.
As expected, in line with previous studies, a moderate weight loss improved all metabolic variables.2, 3, 4, 6 Insulin resistance, as estimated by HOMA index, was significantly improved after weight loss in our subjects.
Insulin resistance could result from the contribution of visceral fat through increased flux of free fatty acids to the liver35 or by the activity of some adipokines, such as leptin and adiponectin36 or by intracellular fatty acid metabolism through activation of serine kinase cascade.37 In agreement with some6 but not all studies,38, 39 adiponectin levels were not modified by moderate weight loss in our subjects, thus adiponectin changes did not seems to have a major role at least in our study in causing the observed improvement of insulin resistance.
On the contrary, it is possible to hypothesize that the significant changes in liver, pancreatic fat content, visceral AT and leptin, observed after weight loss could be all associated with the improvement in insulin resistance.
To test the joint effects of changes in liver and pancreas lipid content, fat distribution, leptin and hs-CRP after weight loss on HOMA improvement we performed a step-down multiple regression analyses: changes in liver lipid content were independently associated with HOMA improvement.
Changes in liver lipid content together with gender explained in our study 55.4% of the variance of insulin resistance improvement after weight loss, independently of visceral fat and pancreas lipid content decrease. This finding seems to be in line with recent observations that increased fat accumulation in liver is the main determinant of peripheral and hepatic insulin resistance40 as well as with the findings that early and major decrease in liver fat after weight loss occurs at the same time of normalization of hepatic insulin sensitivity and fall of glucose levels.41
Some limitations of our study should be recognized. First, the relatively small study sample size. Further, although MR spectroscopy has been used in the majority of studies that evaluated liver lipid content before and after weight loss, we used the MR chemical shift technique. However, high accuracy for the quantification of liver lipid content, validated also against histological determined percentage of fat,22 and pancreas lipid content20 has been recently reported for this technique. Moreover, it has been recently shown that in/opposed phase technique is a valid and reliable tool for both, hepatic and pancreatic fat quantification, showing good agreement with fat-selective spectral-spatial gradient-echo imaging20, 42, 43 and also with magnetic resonance spectroscopy.44 MR spectroscopy still lacks general availability in current clinical practice, and the analysis can be performed only on one voxel at a time. On the contrary, MRI scan using T1-weighted gradient-echo in-phase and opposed-phase sequence is a rapid and available technique, already routinely included in MR-imaging protocols for the upper abdomen. Moreover, the analysis can be performed on whole organs, and not on single voxels.
Finally, we only evaluated a surrogate marker of insulin resistance such as HOMA index and then we were not able to evaluate hepatic insulin resistance. Thus, it is possible to speculate that our results could be even more relevant if more sophisticated methods tailored to test hepatic insulin resistance were used.
In conclusion, our data show that a moderate weight loss determines a significant decline in visceral abdominal fat, together with a decline in lipid fat content inside liver and pancreas. After weight loss percent changes are greater in liver than in pancreas lipid content and in visceral abdominal AT. Finally, changes in liver lipid content seem to be the most important predictor of insulin resistance improvement after weight loss. These results have clinical implications showing that even a small weight loss is able to reduce ectopic fat deposition in different splanchnic districts (liver and pancreas) and this reduction is significantly related to relevant metabolic improvements and showed that moderate weight loss is a cornerstone treatment of liver steatosis in obese people.
References
Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001; 344: 1343–1350.
Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA et al. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346: 393–403.
Zamboni M, Armellini F, Turcato E, Todesco T, Bissoli L, Bergamo Andreis IA et al. Effect of weight loss on regional body fat distribution in premenopausal women. Am J Clin Nutr 1993; 58: 29–34.
Goodpaster BH, Kelley DE, Wing RR, Meier A, Thaete FL . Effects of weight loss on regional fat distribution and insulin sensitivity in obesity. Diabetes 1999; 48: 839–847.
Montani JP, Carroll JF, Dwyer TM, Antic V, Yang Z, Dulloo AG . Ectopic fat storage in heart, blood vessels and kidneys in the pathogenesis of cardiovascular diseases. Int J Obes 2004; 28 (Suppl 4): S58–S65.
Mazzali G, Di Francesco V, Zoico E, Fantin F, Zamboni G, Benati C et al. Interrelations between fat distribution, muscle lipid content, adipocytokines, and insulin resistance: effect of moderate weight loss in older women. Am J Clin Nutr 2006; 84: 1193–1199.
Dulloo AG, Jacquet J, Solinas G, Montani J-P, Schutz Y . Body composition phenotypes in pathways to obesity and the metabolic syndrome. Int J Obes 2010; 34: S4–S17.
Goodpaster BH, Delany JP, Otto AD, Kuller L, Vockley J, South-Paul JE et al. Effects of diet and physical activity interventions on weight loss and cardiometabolic risk factors in severely obese adults: a randomized trial. JAMA 2010; 304: 1795–1802.
Medrikova D, Jilkova ZM, Bardava K, Janovska P, Rossmeisl M, Kopecky J . Sex differences during the course of diet-induced obesity in mice: adipose tissue expandability and glycemic control. Int J Obes 2011; 36: 1–11.
Lee Y, Lingvay I, Szczepaniak LS, Ravazzola M, Orci L, Unger RH . Pancreatic steatosis: harbinger of type 2 diabetes in obese rodents. Int J Obes 2010; 34: 396–400.
Colles SL, Dixon JB, Marks P, Strauss BJ, O'Brien PE . Preoperative weight loss with a very low energy diet: quantitation of changes in liver and abdominal fat by serial imaging. Am J Clin Nutr 2006; 84: 304–311.
Petersen KF, Dufour S, Befroy D, Lehrke M, Hendler RE, Shulman GI . Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance and hyperglycemia by moderate weight reduction in patients with type 2 of diabetes. Diabetes 2005; 54: 603–608.
Ross R, Shaw KD, Martel Y, de Guise J, Avruch L . Adipose tissue distribution measured by magnetic resonance imaging in obese women. Am J Clin Nutr 1993; 57: 470–475.
The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 2003; 26: 3160–3167.
Angulo P . Nonhalcoholic fatty liver disease. New Eng J Med 2002; 346: 1221–1231.
Pietrobelli A, Formica C, Wang Z, Heymsfield SB . Dual-energy X-ray absorptiometry body composition model: review of physical concepts. Am J Physiol 1996; 271: E941–E951.
Rossi AP, Fantin F, Zamboni G, Mazzali G, Rinaldi CA, Del Giglio M et al. Predictors of ectopic fat deposition in liver and pancreas in obese men and women. Obesity 2011; 19: 1747–1754.
Qayyum A, Goh JS, Kakar S, Yeh BM, Merriman RB, Coakley FV . Accuracy of liver fat quantification at MR imaging: comparison of out-of-phase gradient-echo and fat-saturated fast spin-echo techniques—initial experience. Radiology 2005; 237: 507–511.
Kim HJ, Byun JH, Park SH, Shin YM, Kim PN, Ha HK et al. Focal fatty replacement of the pancreas: usefulness of chemical shift MRI. Am J Roentgenol 2007; 188: 429–432.
Lee SE, Jang J-Y, Lim C-S, Kang MJ, Kim SH, Kim MA et al. Measurement of pancreatic fat by magnetic resonance imaging. Predicting the occurrence of pancreatic fistula after pancreatoduodenectomy. Ann Surg 2010; 251: 932–936.
Brunt EM . Nonalcoholic steatohepatitis: definition and pathology. Semin Liver Dis 2001; 21: 3–16.
Bahl M, Qayyum A, Westphalen AC, Noworolski SM, Chu PW, Ferrell L et al. Liver steatosis: investigation of opposed-phase T1-weighted liver MR signal intensity loss and visceral fat measurement as biomarkers. Radiology 2008; 249: 160–166.
Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care 2000; 23: 57–63.
Istituto Nazionale della Nutrizione. Tabelle di composizione degli alimenti. (National Institute of Nutrition: tables of nutritional composition.). Litho Delta: Milano, 1989 (in Italian).
SPSS Inc. SPSS-X User's Guide 2nd edn McGraw-Hill: New York, NY, 1986.
Chaston TB, Dixon JB . Factors associated with percent change in visceral versus subcutaneous abdominal fat during weight loss: findings from a systematic review. Int J Obes 2008; 32: 619–628.
Tamura Y, Tanaka Y, Sato F, Choi, Watada H, Niwa M et al. Effects of diet and exercise on muscle and liver intracellular lipid contents and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab 2005; 90: 3191–3196.
Larson-Meyer DE, Heilbronn LK, Redman LM, Newcomer BR, Frisard MI, Anton S et al. Effect of calorie restriction with or without exercise on insulin sensitivity, beta cell function, fat cell size and ectopic lipid in overweight subjects. Diabetes Care 2006; 29: 1337–1344.
Sato F, Tamura Y, Watada H, Kumashiro N, Igarashi Y, Uchino H et al Effects of diet-induced moderate weight reduction on intrahepatic and intramyocellular triglycerides and glucose metabolism in obese subjects. J Clin Endocrinol Metab 2007; 92: 3326–3329.
Shah K, Stufflebam A, Hilton TN, Sinacore DR, Klein S, Villareal DT . Diet and exercise interventions reduce intrahepatic fat content and improve insulin sensitivity in obese older adults. Obesity 2009; 17: 2162–2168.
Tushuizen ME, Bunck MC, Pouwels PJ, Bontemps S, van Waesberghe JH, Schindhelm RK et al. Pancreatic fat content and Beta cell function in men with and without type 2 diabetes. Diabetes Care 2007; 30: 2916–2921.
Unger RH . Reinventing type 2 diabetes. Pathogenesis, treatment and prevention. JAMA 2008; 299: 1185–1187.
Lee JS, Kim SH, Jun DW, Han JH, Jang EC, Park JY et al. Clinical implications of fatty pancreas: correlations between fatty pancreas and metabolic syndrome. World J Gastroenterol 2009; 15: 1869–1875.
Pinnick KE, Collins SC, Londos C, Gauguier D, Clark A, Fielding BA . Pancreatic ectopic fati is characterized by adipocyte infiltration and altered lipid composition. Obesity 2008; 16: 522–530.
Bosello O, Zamboni M . Visceral obesity and metabolic sindrome. Obes Rev 2000; 1: 47–56.
Arner P . The adipocyte in insulin resistance: key molecules and the impact of the thiazolidinediones. Trend Endocrinol Metab 2003; 14: 137–145.
Shulman GI . Cellular mechanisms of insulin resistance. J Clin Invest 2000; 106: 171–176.
Yang WS, Lee WJ, Funahashi T, Tanaka S, Matsuzawa Y, Chao CL et al. Weight reduction increases plasma levels of an adipose-derived anti-inflammatory protein, adiponectin. J Clin Endocrinol Metab 2001; 86: 3815–3819.
Monzillo LU, Hamdy O, Horton ES, Ledbury S, Mullooly C, Jarema C et al. Effects of lifestyle modification on adipokine levels in obese subjects with insulin resistance. Obes Res 2003; 11: 1048–1054.
Fabbrini E, Magkos F, Mohammed BS, Pietka T, Abumrad NA, Patterson BW et al. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci 2009; 106: 15430–15435.
Taylor R . Pathogenesis of type 2 diabetes: tracing the reverse route from cure to cause. Diabetologia 2008; 51: 1781–1789.
Schwenzer NF, Machann J, Martirosian P, Stefan N, Schraml C, Fritsche A et al. Quantification of pancreatic lipomatosis and liver steatosis by MRI: comparison of in/opposed- phase and spectral-spatial exitation techniques. Invest Radiol 2008; 43: 330–337.
Le KA, Ventura EE, Fisher JQ, Davis JN, Weigensberg MJ, Punyanitya M et al. Ethnic differences in pancreatic fat accumulation and its relationship with other fat depots and inflammatory markers. Diabetes Care 2011; 34: 485–490.
Sijens PE, Edens MA, Bakker SJL, Stolk RP . MRI-determined fat content of human liver, pancreas and kidney. World J Gastroenterol 2010; 16: 1992–1998.
Acknowledgements
The research was supported by Italian Minister of Health project ‘Pathophysiology, clinical and therapeutical aspects of morbid obesity: a comprehensive approach in the management of the disease and its comorbidities’.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Rossi, A., Fantin, F., Zamboni, G. et al. Effect of moderate weight loss on hepatic, pancreatic and visceral lipids in obese subjects. Nutr & Diabetes 2, e32 (2012). https://doi.org/10.1038/nutd.2012.5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nutd.2012.5
Keywords
This article is cited by
-
Comparisons of calorie restriction and structured exercise on reductions in visceral and abdominal subcutaneous adipose tissue: a systematic review
European Journal of Clinical Nutrition (2022)