Abstract
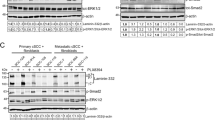
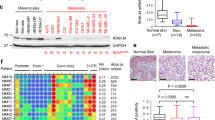
Inactivation of the p16INK4a tumor suppressor protein is critical for the development of human cancers, including human melanoma. However, the molecular basis of the protein's inhibitory effect on cancer development is not clear. Here we investigated a possible mechanism for p16INK4a inhibition of neoplastic transformation and UV-induced skin cancer. We show that p16INK4a suppresses the activity of c-Jun N-terminal kinases (JNKs) and that it binds to the glycine-rich loop of the N-terminal domain of JNK3. Although p16INK4a does not affect the phosphorylation of JNKs, its interaction with JNK inhibits c-Jun phosphorylation induced by UV exposure. This, in turn, interferes with cell transformation promoted by the H-Ras–JNK–c-Jun–AP-1 signaling axis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Xiong, Y., Zhang, H. & Beach, D. Subunit rearrangement of the cyclin-dependent kinases is associated with cellular transformation. Genes Dev. 7, 1572–1583 (1993).
Sherr, C.J. Parsing Ink4a/Arf: “pure” p16-null mice. Cell 106, 531–534 (2001).
Caldas, C. et al. Frequent somatic mutations and homozygous deletions of the p16 (MTS1) gene in pancreatic adenocarcinoma. Nat. Genet. 8, 27–32 (1994).
Serrano, M., Gomez-Lahoz, E., DePinho, R.A., Beach, D. & Bar-Sagi, D. Inhibition of ras-induced proliferation and cellular transformation by p16INK4. Science 267, 249–252 (1995).
Serrano, M. et al. Role of the INK4a locus in tumor suppression and cell mortality. Cell 85, 27–37 (1996).
Krimpenfort, P., Quon, K.C., Mooi, W.J., Loonstra, A. & Berns, A. Loss of p16Ink4a confers susceptibility to metastatic melanoma in mice. Nature 413, 83–86 (2001).
Zhang, H. & Rosdahl, I. Deletion in p16INK4a and loss of p16 expression in human skin primary and metastatic melanoma cells. Int. J. Oncol. 24, 331–335 (2004).
Flores, J.F. et al. Loss of the p16INK4a and p15INK4b genes, as well as neighboring 9p21 markers, in sporadic melanoma. Cancer Res. 56, 5023–5032 (1996).
Walker, G.J. et al. Virtually 100% of melanoma cell lines harbor alterations at the DNA level within CDKN2A, CDKN2B, or one of their downstream targets. Genes Chromosom. Cancer 22, 157–163 (1998).
Bode, A.M. & Dong, Z. Mitogen-activated protein kinase activation in UV-induced signal transduction. Sci. STKE 2003, RE2 (2003).
Sharpless, E. & Chin, L. The INK4a/ARF locus and melanoma. Oncogene 22, 3092–3098 (2003).
Recio, J.A. et al. Ink4a/arf deficiency promotes ultraviolet radiation-induced melanomagenesis. Cancer Res. 62, 6724–6730 (2002).
Noonan, F.P. et al. Neonatal sunburn and melanoma in mice. Nature 413, 271–272 (2001).
Davis, R.J. The mitogen-activated protein kinase signal transduction pathway. J. Biol. Chem. 268, 14553–14556 (1993).
Treisman, R. Regulation of transcription by MAP kinase cascades. Curr. Opin. Cell Biol. 8, 205–215 (1996).
Davis, R.J. Signal transduction by the JNK group of MAP kinases. Cell 103, 239–252 (2000).
Ip, Y.T. & Davis, R.J. Signal transduction by the c-Jun N-terminal kinase (JNK)–from inflammation to development. Curr. Opin. Cell Biol. 10, 205–219 (1998).
Huang, C., Li, J., Ma, W.Y. & Dong, Z. JNK activation is required for JB6 cell transformation induced by tumor necrosis factor-α but not by 12-O-tetradecanoylphorbol-13-acetate. J. Biol. Chem. 274, 29672–29676 (1999).
Chen, N. et al. Suppression of skin tumorigenesis in c-Jun NH(2)-terminal kinase-2-deficient mice. Cancer Res. 61, 3908–3912 (2001).
She, Q.B., Chen, N., Bode, A.M., Flavell, R.A. & Dong, Z. Deficiency of c-Jun-NH(2)-terminal kinase-1 in mice enhances skin tumor development by 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 62, 1343–1348 (2002).
Dong, Z., Birrer, M.J., Watts, R.G., Matrisian, L.M. & Colburn, N.H. Blocking of tumor promoter-induced AP-1 activity inhibits induced transformation in JB6 mouse epidermal cells. Proc. Natl. Acad. Sci. USA 91, 609–613 (1994).
Young, M.R. et al. Transgenic mice demonstrate AP-1 (activator protein-1) transactivation is required for tumor promotion. Proc. Natl. Acad. Sci. USA 96, 9827–9832 (1999).
Zenz, R. et al. c-Jun regulates eyelid closure and skin tumor development through EGFR signaling. Dev. Cell 4, 879–889 (2003).
Derijard, B. et al. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell 76, 1025–1037 (1994).
Kallunki, T. et al. JNK2 contains a specificity-determining region responsible for efficient c-Jun binding and phosphorylation. Genes Dev. 8, 2996–3007 (1994).
Katchalski-Katzir, E. et al. Molecular surface recognition: determination of geometric fit between proteins and their ligands by correlation techniques. Proc. Natl. Acad. Sci. USA 89, 2195–2199 (1992).
Vakser, I.A. & Aflalo, C. Hydrophobic docking: a proposed enhancement to molecular recognition techniques. Proteins 20, 320–329 (1994).
Byeon, I.J. et al. Tumor suppressor p16INK4A: determination of solution structure and analyses of its interaction with cyclin-dependent kinase 4. Mol. Cell 1, 421–431 (1998).
Moran, M.F., Polakis, P., McCormick, F., Pawson, T. & Ellis, C. Protein-tyrosine kinases regulate the phosphorylation, protein interactions, subcellular distribution, and activity of p21ras GTPase-activating protein. Mol. Cell. Biol. 11, 1804–1812 (1991).
Sachsenmaier, C. et al. Involvement of growth factor receptors in the mammalian UVC response. Cell 78, 963–972 (1994).
Khosravi-Far, R. et al. Oncogenic Ras activation of Raf/mitogen-activated protein kinase-independent pathways is sufficient to cause tumorigenic transformation. Mol. Cell. Biol. 16, 3923–3933 (1996).
Campbell, I., Magliocco, A., Moyana, T., Zheng, C. & Xiang, J. Adenovirus-mediated p16INK4 gene transfer significantly suppresses human breast cancer growth. Cancer Gene Ther. 7, 1270–1278 (2000).
Quelle, D.E., Zindy, F., Ashmun, R.A. & Sherr, C.J. Alternative reading frames of the INK4a tumor suppressor gene encode two unrelated proteins capable of inducing cell cycle arrest. Cell 83, 993–1000 (1995).
Maestro, R. & Boiocchi, M. Sunlight and melanoma: an answer from MTS1 (p16). Science 267, 15–16 (1995).
Pollock, P.M., Yu, F., Qiu, L., Parsons, P.G. & Hayward, N.K. Evidence for u.v. induction of CDKN2 mutations in melanoma cell lines. Oncogene 11, 663–668 (1995).
Kennedy, N.J. & Davis, R.J. Role of JNK in tumor development. Cell Cycle 2, 199–201 (2003).
Kurokawa, M. et al. The evi-1 oncoprotein inhibits c-Jun N-terminal kinase and prevents stress-induced cell death. EMBO J. 19, 2958–2968 (2000).
Adler, V. et al. Regulation of JNK signaling by GSTp. EMBO J. 18, 1321–1334 (1999).
Russo, A.A., Tong, L., Lee, J.O., Jeffrey, P.D. & Pavletich, N.P. Structural basis for inhibition of the cyclin-dependent kinase Cdk6 by the tumour suppressor p16INK4a. Nature 395, 237–243 (1998).
Angel, P., Szabowski, A. & Schorpp-Kistner, M. Function and regulation of AP-1 subunits in skin physiology and pathology. Oncogene 20, 2413–2423 (2001).
Shaulian, E. & Karin, M. AP-1 in cell proliferation and survival. Oncogene 20, 2390–2400 (2001).
Berggren, P. et al. Detecting homozygous deletions in the CDKN2A(p16(INK4a))/ARF(p14(ARF)) gene in urinary bladder cancer using real-time quantitative PCR. Clin. Cancer Res. 9, 235–242 (2003).
Zhong, S. et al. Ultraviolet B-induced phosphorylation of histone H3 at serine 28 is mediated by MSK1. J. Biol. Chem. 276, 33213–33219 (2001).
Vakser, I.A. Protein docking for low-resolution structures. Protein Eng. 8, 371–377 (1995).
Vakser, I.A., Matar, O.G. & Lam, C.F. A systematic study of low-resolution recognition in protein–protein complexes. Proc. Natl. Acad. Sci. USA 96, 8477–8482 (1999).
Clark, G.J., Cox, A.D., Graham, S.M. & Der, C.J. Biological assays for Ras transformation. Methods Enzymol. 255, 395–412 (1995).
Acknowledgements
This work was supported in part by the Hormel Foundation and grants from the US National Institutes of Health. We thank R. Davis for JNKs plasmids, C. Chen and Z. Kiss for Rb−/− and CHO-K1 cell lines and A. Hansen for secretarial assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Specific binding assay of JNK1 or JNK3 and p16INK4a. (PDF 88 kb)
Supplementary Fig. 2
Sequence similarity of JNKs and interaction with p16INK4a. (PDF 1694 kb)
Rights and permissions
About this article
Cite this article
Choi, B., Choi, H., Ko, K. et al. The tumor suppressor p16INK4a prevents cell transformation through inhibition of c-Jun phosphorylation and AP-1 activity. Nat Struct Mol Biol 12, 699–707 (2005). https://doi.org/10.1038/nsmb960
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb960
This article is cited by
-
TGF-β1/IL-11/MEK/ERK signaling mediates senescence-associated pulmonary fibrosis in a stress-induced premature senescence model of Bmi-1 deficiency
Experimental & Molecular Medicine (2020)
-
The role of tumor suppressor p15Ink4b in the regulation of hematopoietic progenitor cell fate
Blood Cancer Journal (2013)
-
Jnk3
AfCS-Nature Molecule Pages (2011)
-
The p16INK4A tumor suppressor regulates cellular oxidative stress
Oncogene (2011)
-
Cyclin-dependent kinase 1 expression is inhibited by p16INK4a at the post-transcriptional level through the microRNA pathway
Oncogene (2011)