Abstract
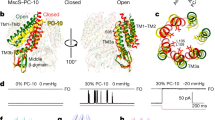
We describe a mechanism connecting the adaptive behavior of the bacterial mechanosensitive channel MscS to the flexibility of the pore-lining helix TM3. Simulated expansion of the channel structure revealed straightening of a characteristic kink near Gly113 in the open state; return to the closed state produced an alternative kink at Gly121. Patch-clamp experiments showed that higher helical propensity introduced by a G113A mutation prevented inactivation. A similar mutation, G121A, kinetically impeded both closure and inactivation. Duplicating the glycines at each of these sites to increase flexibility produced directly opposite effects. The severely toxic G113A G121A mutation resulted in channels that could not inactivate or close with the release of tension. These data suggest that the open MscS features straight TM3 helices, which act as collapsible 'struts'. Closure and desensitization rely on buckling at Gly121, whereas the crystal-like kink at Gly113 is a feature of the inactivated state.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Levina, N. et al. Protection of Escherichia coli cells against extreme turgor by activation of MscS and MscL mechanosensitive channels: identification of genes required for MscS activity. EMBO J. 18, 1730–1737 (1999).
Pivetti, C.D. et al. Two families of mechanosensitive channel proteins. Microbiol. Mol. Biol. Rev. 67, 66–85 (2003).
Nakayama, Y., Fujiu, K., Sokabe, M. & Yoshimura, K. Molecular and electrophysiological characterization of a mechanosensitive channel expressed in the chloroplasts of Chlamydomonas. Proc. Natl. Acad. Sci. USA 104, 5883–5888 (2007).
Haswell, E.S. & Meyerowitz, E.M. MscS-like proteins control plastid size and shape in Arabidopsis thaliana. Curr. Biol. 16, 1–11 (2006).
Sukharev, S.I., Martinac, B., Arshavsky, V.Y. & Kung, C. Two types of mechanosensitive channels in the Escherichia coli cell envelope: solubilization and functional reconstitution. Biophys. J. 65, 177–183 (1993).
Sukharev, S. Purification of the small mechanosensitive channel of Escherichia coli (MscS): the subunit structure, conduction, and gating characteristics in liposomes. Biophys. J. 83, 290–298 (2002).
Okada, K., Moe, P.C. & Blount, P. Functional design of bacterial mechanosensitive channels. Comparisons and contrasts illuminated by random mutagenesis. J. Biol. Chem. 277, 27682–27688 (2002).
Miller, S. et al. Domain organization of the MscS mechanosensitive channel of Escherichia coli. EMBO J. 22, 36–46 (2003).
Akitake, B., Anishkin, A. & Sukharev, S. The “dashpot” mechanism of stretch-dependent gating in MscS. J. Gen. Physiol. 125, 143–154 (2005).
Koprowski, P. & Kubalski, A. Voltage-independent adaptation of mechanosensitive channels in Escherichia coli protoplasts. J. Membr. Biol. 164, 253–262 (1998).
Edwards, M.D., Booth, I.R. & Miller, S. Gating the bacterial mechanosensitive channels: MscS a new paradigm? Curr. Opin. Microbiol. 7, 163–167 (2004).
Edwards, M.D. et al. Pivotal role of the glycine-rich TM3 helix in gating the MscS mechanosensitive channel. Nat. Struct. Mol. Biol. 12, 113–119 (2005).
Bass, R.B., Strop, P., Barclay, M. & Rees, D.C. Crystal structure of Escherichia coli MscS, a voltage-modulated and mechanosensitive channel. Science 298, 1582–1587 (2002).
Anishkin, A. & Sukharev, S. Water dynamics and dewetting transitions in the small mechanosensitive channel MscS. Biophys. J. 86, 2883–2895 (2004).
Sotomayor, M. & Schulten, K. Molecular dynamics study of gating in the mechanosensitive channel of small conductance MscS. Biophys. J. 87, 3050–3065 (2004).
Sotomayor, M., van der Straaten, T.A., Ravaioli, U. & Schulten, K. Electrostatic properties of the mechanosensitive channel of small conductance MscS. Biophys. J. 90, 3496–3510 (2006).
Spronk, S.A., Elmore, D.E. & Dougherty, D.A. Voltage-dependent hydration and conduction properties of the hydrophobic pore of the mechanosensitive channel of small conductance. Biophys. J. 90, 3555–3569 (2006).
Sotomayor, M., Vasquez, V., Perozo, E. & Schulten, K. Ion conduction through MscS as determined by electrophysiology and simulation. Biophys. J. 92, 886–902 (2006).
Schumann, U., Edwards, M.D., Li, C. & Booth, I.R. The conserved carboxy-terminus of the MscS mechanosensitive channel is not essential but increases stability and activity. FEBS Lett. 572, 233–237 (2004).
Koprowski, P. & Kubalski, A. C termini of the Escherichia coli mechanosensitive ion channel (MscS) move apart upon the channel opening. J. Biol. Chem. 278, 11237–11245 (2003).
Miller, S., Edwards, M.D., Ozdemir, C. & Booth, I.R. The closed structure of the MscS mechanosensitive channel. Cross-linking of single cysteine mutants. J. Biol. Chem. 278, 32246–32250 (2003).
Anishkin, A., Akitake, B. & Sukharev, S. Characterization of the resting MscS: modeling and analysis of the closed bacterial mechanosensitive channel of small conductance. Biophys. J., published online 2 November 2007 (doi:10.1529/biophysj.107.110171).
Simons, K.T., Bonneau, R., Ruczinski, I. & Baker, D. Ab initio protein structure prediction of CASP III targets using ROSETTA. Proteins Suppl. 3, 171–176 (1999).
Simons, K.T., Strauss, C. & Baker, D. Prospects for ab initio protein structural genomics. J. Mol. Biol. 306, 1191–1199 (2001).
Simons, K.T., Kooperberg, C., Huang, E. & Baker, D. Assembly of protein tertiary structures from fragments with similar local sequences using simulated annealing and Bayesian scoring functions. J. Mol. Biol. 268, 209–225 (1997).
Lomize, A.L., Pogozheva, I.D., Lomize, M.A. & Mosberg, H.I. Positioning of proteins in membranes: a computational approach. Protein Sci. 15, 1318–1333 (2006).
Jiang, Y. et al. Crystal structure and mechanism of a calcium-gated potassium channel. Nature 417, 515–522 (2002).
Long, S.B., Campbell, E.B. & Mackinnon, R. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science 309, 897–903 (2005).
Shealy, R.T., Murphy, A.D., Ramarathnam, R., Jakobsson, E. & Subramaniam, S. Sequence-function analysis of the K+-selective family of ion channels using a comprehensive alignment and the KcsA channel structure. Biophys. J. 84, 2929–2942 (2003).
Kellenberger, S., West, J.W., Catterall, W.A. & Scheuer, T. Molecular analysis of potential hinge residues in the inactivation gate of brain type IIA Na+ channels. J. Gen. Physiol. 109, 607–617 (1997).
Ding, S., Ingleby, L., Ahern, C.A. & Horn, R. Investigating the putative glycine hinge in Shaker potassium channel. J. Gen. Physiol. 126, 213–226 (2005).
Magidovich, E. & Yifrach, O. Conserved gating hinge in ligand- and voltage-dependent K+ channels. Biochemistry 43, 13242–13247 (2004).
Liu, L.P. & Deber, C.M. Uncoupling hydrophobicity and helicity in transmembrane segments. Alpha-helical propensities of the amino acids in non-polar environments. J. Biol. Chem. 273, 23645–23648 (1998).
Serrano, L., Neira, J.L., Sancho, J. & Fersht, A.R. Effect of alanine versus glycine in alpha-helices on protein stability. Nature 356, 453–455 (1992).
Li, Y., Moe, P.C., Chandrasekaran, S., Booth, I.R. & Blount, P. Ionic regulation of MscK, a mechanosensitive channel from Escherichia coli. EMBO J. 21, 5323–5330 (2002).
Ou, X., Blount, P., Hoffman, R.J. & Kung, C. One face of a transmembrane helix is crucial in mechanosensitive channel gating. Proc. Natl. Acad. Sci. USA 95, 11471–11475 (1998).
Liu, L.P. & Deber, C.M. Guidelines for membrane protein engineering derived from de novo designed model peptides. Biopolymers 47, 41–62 (1998).
Beckstein, O. & Sansom, M.S. Liquid-vapor oscillations of water in hydrophobic nanopores. Proc. Natl. Acad. Sci. USA 100, 7063–7068 (2003).
Zhao, Y., Yarov-Yarovoy, V., Scheuer, T. & Catterall, W.A. A gating hinge in Na+ channels; a molecular switch for electrical signaling. Neuron 41, 859–865 (2004).
Phillips, J.C. et al. Scalable molecular dynamics with NAMD. J. Comput. Chem. 26, 1781–1802 (2005).
MacKerell, A.D. et al. All-atom empirical potential for molecular modeling and dynamics studies of proteins. J. Phys. Chem. B 102, 3586–3616 (1998).
Humphrey, W., Dalke, A. & Schulten, K. VMD: visual molecular dynamics. J. Mol. Graph. 14, 33–8–27–8 (1996).
Jorgensen, W.L., Chandrasekhar, J. & Madura, J.D. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79, 926–935 (1983).
Cui, C., Smith, D.O. & Adler, J. Characterization of mechanosensitive channels in Escherichia coli cytoplasmic membrane by whole-cell patch clamp recording. J. Membr. Biol. 144, 31–42 (1995).
Martinac, B., Buechner, M., Delcour, A.H., Adler, J. & Kung, C. Pressure-sensitive ion channel in Escherichia coli. Proc. Natl. Acad. Sci. USA 84, 2297–2301 (1987).
Acknowledgements
We thank L. Shirinian for generating some of the MscS mutants, M. Kiyatkin for compiling a library of MscS and MscK homologs and conducting the multiple sequence alignments, and I.R. Booth (University of Aberdeen) and P. Blount (University of Texas, Dallas) for kindly providing the MJF465 and PB113 strains. This work was supported by the US National Institutes of Health.
Author information
Authors and Affiliations
Contributions
B.A., A.A. and S.S. designed the experiments and analyzed the models and data. B.A. conducted all of the patch-clamp experiments and generated some of the MscS mutants. A.A. performed all of the computations (extrapolated-motion analysis and molecular dynamics simulations). N.L. performed the growth-curve analysis and generated some of the MscS mutants. The paper was written and edited by B.A., A.A. and S.S.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6, Supplementary Methods (PDF 663 kb)
Rights and permissions
About this article
Cite this article
Akitake, B., Anishkin, A., Liu, N. et al. Straightening and sequential buckling of the pore-lining helices define the gating cycle of MscS. Nat Struct Mol Biol 14, 1141–1149 (2007). https://doi.org/10.1038/nsmb1341
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb1341
This article is cited by
-
Structural mechanism for gating of a eukaryotic mechanosensitive channel of small conductance
Nature Communications (2020)
-
Evolutionary specialization of MscCG, an MscS-like mechanosensitive channel, in amino acid transport in Corynebacterium glutamicum
Scientific Reports (2018)
-
Spatiotemporal relationships defining the adaptive gating of the bacterial mechanosensitive channel MscS
European Biophysics Journal (2018)
-
The role of MscL amphipathic N terminus indicates a blueprint for bilayer-mediated gating of mechanosensitive channels
Nature Communications (2016)
-
The role of lipids in mechanosensation
Nature Structural & Molecular Biology (2015)