Abstract
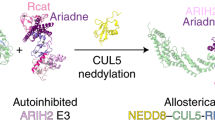
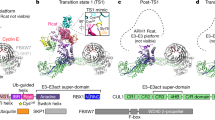
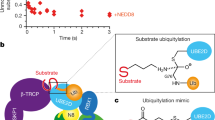
Although cullin-1 neddylation is crucial for the activation of SCF ubiquitin E3 ligases, the underlying mechanisms for NEDD8-mediated activation of SCF remain unclear. Here we demonstrate by NMR and mutational studies that NEDD8 binds the ubiquitin E2 (UBC4), but not NEDD8 E2 (UBC12). Our data imply that NEDD8 forms an active platform on the SCF complex for selective recruitment of ubiquitin-charged E2s in collaboration with RBX1, and thereby upregulates the E3 activity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Petroski, M.D. & Deshaies, R.J. Nat. Rev. Mol. Cell Biol. 6, 9–20 (2005).
Kerscher, O., Felberbaum, R. & Hochstrasser, M. Annu. Rev. Cell Dev. Biol. 22, 159–180 (2006).
Pan, Z.Q., Kentsis, A., Dias, D.C., Yamoah, K. & Wu, K. Oncogene 23, 1985–1997 (2004).
Wu, J.T., Chan, Y.R. & Chien, C.T. Trends Cell Biol. 16, 362–369 (2006).
Parry, G. & Estelle, M. Semin. Cell Dev. Biol. 15, 221–229 (2004).
Wei, N. & Deng, X.W. Annu. Rev. Cell Dev. Biol. 19, 261–286 (2003).
Kawakami, T. et al. EMBO J. 20, 4003–4012 (2001).
Wu, K., Chen, A., Tan, P. & Pan, Z.Q. J. Biol. Chem. 277, 516–527 (2002).
Zheng, N. et al. Nature 416, 703–709 (2002).
Zheng, N., Wang, P., Jeffrey, P.D. & Pavletich, N.P. Cell 102, 533–539 (2000).
Megumi, Y. et al. Genes Cells 10, 679–691 (2005).
Acknowledgements
We thank T. Kasuya, Y. Kito, K. Senda and K. Hattori for their help in the preparation of recombinant proteins. Work in the laboratory of K.K. and Y.Y. was supported by a Grant-in-Aid for Scientific Research on Priority Areas (17028047 and 18076003) from the Ministry of Education, Culture, Sports, Science and Technology, Japan, and by a Grant-in-Aid for Scientific Research (B) (18390016) from Japan Society for the Promotion of Science. E.S. is a recipient of Japan Society for the Promotion of Science Research Fellowships for Young Scientists.
Author information
Authors and Affiliations
Contributions
K.K. contributed to overall guidance of the project. E.S., Y.Y. and K.K. contributed to the design and execution of the NMR study. E.S., K.I., T.C. and K.T. contributed to the design of the mutational studies. E.S., Y.M., Y.S. and N.M. contributed to the execution of the mutational studies. E.S. and K.K. wrote the manuscript. K.I., T.C. and K.T. commented on the manuscript. All authors edited and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Chemical shift perturbation data for NEDD8-E2 interactions. (PDF 147 kb)
Supplementary Fig. 2
Chemical shift perturbation of UBC4 upon binding to wild-type and I44A-mutated NEDD8. (PDF 212 kb)
Supplementary Fig. 3
Evaluation of E2 activities of the UBC4 mutants in in vitro autoubiquitination of MBP-RMA1 and ubiquitination of Sic1PY working with RSP5. (PDF 232 kb)
Supplementary Fig. 4
Effects of the I44A mutation on in vitro neddylation of cullin-2 and ubiquitination of the MBP-ODD. (PDF 81 kb)
Supplementary Fig. 5
Sequence alignment of human UBC4, UBCH7 and UBC12. (PDF 116 kb)
Supplementary Fig. 6
A working model of the neddylated SCFβ–TrCP1–UBC4 complex. (PDF 194 kb)
Supplementary Fig. 7
Effects of neddylation on in vitro conjugation of phosphorylated IκBα with methylated ubiquitin. (PDF 73 kb)
Rights and permissions
About this article
Cite this article
Sakata, E., Yamaguchi, Y., Miyauchi, Y. et al. Direct interactions between NEDD8 and ubiquitin E2 conjugating enzymes upregulate cullin-based E3 ligase activity. Nat Struct Mol Biol 14, 167–168 (2007). https://doi.org/10.1038/nsmb1191
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb1191