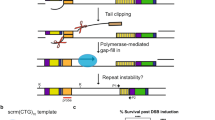
Abstract
Expansions of (CAG)n/(CTG)n trinucleotide repeats are responsible for over a dozen neuromuscular and neurodegenerative disorders. Large-scale expansions are commonly observed in human pedigrees and may be explained by iterative small-scale events such as strand slippage during replication or repair DNA synthesis. Alternatively, a distinct mechanism may lead to a large-scale repeat expansion as a single step. To distinguish between these possibilities, we developed a novel experimental system specifically tuned to analyze large-scale expansions of (CAG)n/(CTG)n repeats in Saccharomyces cerevisiae. The median size of repeat expansions was ∼60 triplets, although we also observed additions of more than 150 triplets. Genetic analysis revealed that Rad51, Rad52, Mre11, Pol32, Pif1, and Mus81 and/or Yen1 proteins are required for large-scale expansions, whereas proteins previously implicated in small-scale expansions are not involved. From these results, we propose a new model for large-scale expansions, which is based on the recovery of replication forks broken at (CAG)n/(CTG)n repeats via break-induced replication.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
La Spada, A.R. & Taylor, J.P. Repeat expansion disease: progress and puzzles in disease pathogenesis. Nat. Rev. Genet. 11, 247–258 (2010).
McMurray, C.T. Mechanisms of trinucleotide repeat instability during human development. Nat. Rev. Genet. 11, 786–799 (2010).
Nance, M.A. & Myers, R.H. Juvenile onset Huntington's disease: clinical and research perspectives. Ment. Retard. Dev. Disabil. Res. Rev. 7, 153–157 (2001).
Thornton, C.A. Myotonic dystrophy. Neurol. Clin. 32, 705–719 (2014).
Kim, J.C. & Mirkin, S.M. The balancing act of DNA repeat expansions. Curr. Opin. Genet. Dev. 23, 280–288 (2013).
Usdin, K., House, N.C. & Freudenreich, C.H. Repeat instability during DNA repair: insights from model systems. Crit. Rev. Biochem. Mol. Biol. 50, 142–167 (2015).
Gacy, A.M., Goellner, G., Juranić, N., Macura, S. & McMurray, C.T. Trinucleotide repeats that expand in human disease form hairpin structures in vitro. Cell 81, 533–540 (1995).
Kerrest, A. et al. SRS2 and SGS1 prevent chromosomal breaks and stabilize triplet repeats by restraining recombination. Nat. Struct. Mol. Biol. 16, 159–167 (2009).
Freudenreich, C.H., Kantrow, S.M. & Zakian, V.A. Expansion and length-dependent fragility of CTG repeats in yeast. Science 279, 853–856 (1998).
Mirkin, S.M. DNA structures, repeat expansions and human hereditary disorders. Curr. Opin. Struct. Biol. 16, 351–358 (2006).
Mirkin, S.M. Expandable DNA repeats and human disease. Nature 447, 932–940 (2007).
Liu, Y. & Wilson, S.H. DNA base excision repair: a mechanism of trinucleotide repeat expansion. Trends Biochem. Sci. 37, 162–172 (2012).
Kovtun, I.V. et al. OGG1 initiates age-dependent CAG trinucleotide expansion in somatic cells. Nature 447, 447–452 (2007).
Concannon, C. & Lahue, R.S. Nucleotide excision repair and the 26S proteasome function together to promote trinucleotide repeat expansions. DNA Repair (Amst.) 13, 42–49 (2014).
Lin, Y. & Wilson, J.H. Transcription-induced CAG repeat contraction in human cells is mediated in part by transcription-coupled nucleotide excision repair. Mol. Cell. Biol. 27, 6209–6217 (2007).
Miret, J.J., Pessoa-Brandão, L. & Lahue, R.S. Orientation-dependent and sequence-specific expansions of CTG/CAG trinucleotide repeats in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 95, 12438–12443 (1998).
Callahan, J.L., Andrews, K.J., Zakian, V.A. & Freudenreich, C.H. Mutations in yeast replication proteins that increase CAG/CTG expansions also increase repeat fragility. Mol. Cell. Biol. 23, 7849–7860 (2003).
Jung, J., van Jaarsveld, M.T., Shieh, S.Y., Xu, K. & Bonini, N.M. Defining genetic factors that modulate intergenerational CAG repeat instability in Drosophila melanogaster. Genetics 187, 61–71 (2011).
Kovtun, I.V. & McMurray, C.T. Trinucleotide expansion in haploid germ cells by gap repair. Nat. Genet. 27, 407–411 (2001).
Wheeler, V.C. et al. Length-dependent gametic CAG repeat instability in the Huntington's disease knock-in mouse. Hum. Mol. Genet. 8, 115–122 (1999).
Bhattacharyya, S. & Lahue, R.S. Saccharomyces cerevisiae Srs2 DNA helicase selectively blocks expansions of trinucleotide repeats. Mol. Cell. Biol. 24, 7324–7330 (2004).
Su, X.A., Dion, V., Gasser, S.M. & Freudenreich, C.H. Regulation of recombination at yeast nuclear pores controls repair and triplet repeat stability. Genes Dev. 29, 1006–1017 (2015).
Debacker, K. et al. Histone deacetylase complexes promote trinucleotide repeat expansions. PLoS Biol. 10, e1001257 (2012).
Viterbo, D., Michoud, G., Mosbach, V., Dujon, B. & Richard, G.F. Replication stalling and heteroduplex formation within CAG/CTG trinucleotide repeats by mismatch repair. DNA Repair (Amst.) 42, 94–106 (2016).
Samadashwily, G.M., Raca, G. & Mirkin, S.M. Trinucleotide repeats affect DNA replication in vivo. Nat. Genet. 17, 298–304 (1997).
Møllersen, L., Rowe, A.D., Larsen, E., Rognes, T. & Klungland, A. Continuous and periodic expansion of CAG repeats in Huntington's disease R6/1 mice. PLoS Genet. 6, e1001242 (2010).
Gomes-Pereira, M. et al. CTG trinucleotide repeat “big jumps”: large expansions, small mice. PLoS Genet. 3, e52 (2007).
Liu, G. et al. Altered replication in human cells promotes DMPK (CTG)n · (CAG)n repeat instability. Mol. Cell. Biol. 32, 1618–1632 (2012).
Liu, G., Chen, X., Bissler, J.J., Sinden, R.R. & Leffak, M. Replication-dependent instability at (CTG) x (CAG) repeat hairpins in human cells. Nat. Chem. Biol. 6, 652–659 (2010).
Nakatani, R., Nakamori, M., Fujimura, H., Mochizuki, H. & Takahashi, M.P. Large expansion of CTG•CAG repeats is exacerbated by MutSβ in human cells. Sci. Rep. 5, 11020 (2015).
Shishkin, A.A. et al. Large-scale expansions of Friedreich's ataxia GAA repeats in yeast. Mol. Cell 35, 82–92 (2009).
Cherng, N. et al. Expansions, contractions, and fragility of the spinocerebellar ataxia type 10 pentanucleotide repeat in yeast. Proc. Natl. Acad. Sci. USA 108, 2843–2848 (2011).
Shah, K.A., McGinty, R.J., Egorova, V.I. & Mirkin, S.M. Coupling transcriptional state to large-scale repeat expansions in yeast. Cell Rep. 9, 1594–1602 (2014).
Dobi, K.C. & Winston, F. Analysis of transcriptional activation at a distance in Saccharomyces cerevisiae. Mol. Cell. Biol. 27, 5575–5586 (2007).
Kang, S., Jaworski, A., Ohshima, K. & Wells, R.D. Expansion and deletion of CTG repeats from human disease genes are determined by the direction of replication in E. coli. Nat. Genet. 10, 213–218 (1995).
Freudenreich, C.H., Stavenhagen, J.B. & Zakian, V.A. Stability of a CTG/CAG trinucleotide repeat in yeast is dependent on its orientation in the genome. Mol. Cell. Biol. 17, 2090–2098 (1997).
Bhattacharyya, S. & Lahue, R.S. Srs2 helicase of Saccharomyces cerevisiae selectively unwinds triplet repeat DNA. J. Biol. Chem. 280, 33311–33317 (2005).
Schmidt, M.H. & Pearson, C.E. Disease-associated repeat instability and mismatch repair. DNA Repair (Amst.) 38, 117–126 (2016).
Kantartzis, A. et al. Msh2-Msh3 interferes with Okazaki fragment processing to promote trinucleotide repeat expansions. Cell Rep. 2, 216–222 (2012).
Sundararajan, R., Gellon, L., Zunder, R.M. & Freudenreich, C.H. Double-strand break repair pathways protect against CAG/CTG repeat expansions, contractions and repeat-mediated chromosomal fragility in Saccharomyces cerevisiae. Genetics 184, 65–77 (2010).
Symington, L.S., Rothstein, R. & Lisby, M. Mechanisms and regulation of mitotic recombination in Saccharomyces cerevisiae. Genetics 198, 795–835 (2014).
Lydeard, J.R., Jain, S., Yamaguchi, M. & Haber, J.E. Break-induced replication and telomerase-independent telomere maintenance require Pol32. Nature 448, 820–823 (2007).
Schulz, V.P. & Zakian, V.A. The saccharomyces PIF1 DNA helicase inhibits telomere elongation and de novo telomere formation. Cell 76, 145–155 (1994).
Wilson, M.A. et al. Pif1 helicase and Polδ promote recombination-coupled DNA synthesis via bubble migration. Nature 502, 393–396 (2013).
Sakofsky, C.J. et al. Translesion polymerases drive microhomology-mediated break-induced replication leading to complex chromosomal rearrangements. Mol. Cell 60, 860–872 (2015).
Anand, R.P., Lovett, S.T. & Haber, J.E. Break-induced DNA replication. Cold Spring Harb. Perspect. Biol. 5, a010397 (2013).
Morrow, D.M., Connelly, C. & Hieter, P. “Break copy” duplication: a model for chromosome fragment formation in Saccharomyces cerevisiae. Genetics 147, 371–382 (1997).
Deem, A. et al. Break-induced replication is highly inaccurate. PLoS Biol. 9, e1000594 (2011).
Mayle, R. et al. DNA REPAIR. Mus81 and converging forks limit the mutagenicity of replication fork breakage. Science 349, 742–747 (2015).
Lee, C.S., Lee, K., Legube, G. & Haber, J.E. Dynamics of yeast histone H2A and H2B phosphorylation in response to a double-strand break. Nat. Struct. Mol. Biol. 21, 103–109 (2014).
Szilard, R.K. et al. Systematic identification of fragile sites via genome-wide location analysis of gamma-H2AX. Nat. Struct. Mol. Biol. 17, 299–305 (2010).
Sogo, J.M., Lopes, M. & Foiani, M. Fork reversal and ssDNA accumulation at stalled replication forks owing to checkpoint defects. Science 297, 599–602 (2002).
Follonier, C., Oehler, J., Herrador, R. & Lopes, M. Friedreich's ataxia-associated GAA repeats induce replication-fork reversal and unusual molecular junctions. Nat. Struct. Mol. Biol. 20, 486–494 (2013).
Richard, G.-F., Goellner, G.M., McMurray, C.T. & Haber, J.E. Recombination-induced CAG trinucleotide repeat expansions in yeast involve the MRE11-RAD50-XRS2 complex. EMBO J. 19, 2381–2390 (2000).
Jankowski, C., Nasar, F. & Nag, D.K. Meiotic instability of CAG repeat tracts occurs by double-strand break repair in yeast. Proc. Natl. Acad. Sci. USA 97, 2134–2139 (2000).
Kim, H.-M. et al. Chromosome fragility at GAA tracts in yeast depends on repeat orientation and requires mismatch repair. EMBO J. 27, 2896–2906 (2008).
Hastings, P.J., Ira, G. & Lupski, J.R. A microhomology-mediated break-induced replication model for the origin of human copy number variation. PLoS Genet. 5, e1000327 (2009).
Payen, C., Koszul, R., Dujon, B. & Fischer, G. Segmental duplications arise from Pol32-dependent repair of broken forks through two alternative replication-based mechanisms. PLoS Genet. 4, e1000175 (2008).
Higham, C.F., Morales, F., Cobbold, C.A., Haydon, D.T. & Monckton, D.G. High levels of somatic DNA diversity at the myotonic dystrophy type 1 locus are driven by ultra-frequent expansion and contraction mutations. Hum. Mol. Genet. 21, 2450–2463 (2012).
Raca, G., Siyanova, E.Y., McMurray, C.T. & Mirkin, S.M. Expansion of the (CTG)(n) repeat in the 5′-UTR of a reporter gene impedes translation. Nucleic Acids Res. 28, 3943–3949 (2000).
Shah, K.A. et al. Role of DNA polymerases in repeat-mediated genome instability. Cell Rep. 2, 1088–1095 (2012).
Goldstein, A.L. & McCusker, J.H. Three new dominant drug resistance cassettes for gene disruption in Saccharomyces cerevisiae. Yeast 15, 1541–1553 (1999).
Rosche, W.A. & Foster, P.L. Determining mutation rates in bacterial populations. Methods 20, 4–17 (2000).
Aksenova, A.Y. et al. Genome rearrangements caused by interstitial telomeric sequences in yeast. Proc. Natl. Acad. Sci. USA 110, 19866–19871 (2013).
Acknowledgements
Research in the laboratory of S.M.M. is supported by NIH grants R01GM60987 and P01GM105473 and by a generous contribution from the White family. J.C.K. was supported by the NIH Training in Education and Critical Research Skills postdoctoral program (K12GM074869) and by Tufts University. We thank C. Freudenreich for valuable comments on the manuscript, G. Ira and S. Jinks-Robertson for helpful discussions, K. Spivakovsky-Gonzalez for help analyzing MSH mutants, and D. Padmanhaban for help with strain construction. S.T.H. received support from REU award NSF DBI 1263030. T.D. received support from Tufts Summer Scholars.
Author information
Authors and Affiliations
Contributions
J.C.K. and S.M.M. designed the study; J.C.K., S.T.H., and T.D. performed experiments; J.C.K., S.T.H., T.D., and S.M.M. analyzed data; K.A.S. provided reagents; J.C.K. and S.M.M. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
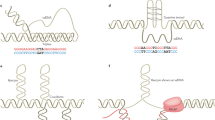
Supplementary Figure 1 Sequence of the cassette used to generate the (CAG)140 strain YJK146.
Gray letters show chromosome III sequences used to target the construct to chromosome III (SGD coordinates 75,227–75,594 at the beginning of the cassette and coordinates 75,641–75,895 at the end). Sequence from the GAL1 promoter is in orange with the four Gal4 binding sites in yellow (Frolova, E. et al., Nucleic Acids Research. 27, 1350–1358, 1999). Other sequences are from the tetracycline resistance gene (green), CAG repeats (lime), TATA box (magenta), CAN1 (turquoise), and TRP1 (blue). Unmarked sequences are from the vector/plasmid.
Supplementary Figure 2 Construction of the (CAG)140 strain used to analyze large-scale CAG-repeat expansions in Saccharomyces cerevisiae.
Spot assay of strains containing no repeats and (CAG)140 with varying distances from UAS (last Gal4 binding site) to TATA box. Spots show 10-fold serial dilutions. Canavanine plate is shown after 5 days of growth at 30°C. Strain marked with red box (YJK146) was used for expansion rate analysis in various gene knockouts.
Supplementary Figure 3 Large-scale CAG-repeat expansions can be recovered and analyzed in budding yeast.
(A) Experimental system to study large-scale CAG repeat expansions. In the starting strain, the distance between the upstream activating sequence (UASGAL) and TATA box promoter (PGAL) allows transcription of the forward selection marker CAN1, which encodes an arginine permease. Large-scale expansions will result in CanR clones. Bars above the CAG repeats indicate products of single colony PCR used for sequencing (PCR1) and determination of repeat length (PCR2). (B) Agarose gel images showing PCR amplification of CAG repeat length for CanR clones. Arrow points to the initial length of (CAG)140. Only expanded clones are used to calculate expansion rates. Asterisks in lanes 2, 3, 6, and 8 indicate expanded clones. Clone 5 may be a large expansion with secondary contractions or the result of a gross chromosomal rearrangement.
Supplementary Figure 4 Genetic control of large-scale CAG-repeat expansions.
Effect of different gene knockouts and mutations on the large-scale expansion rate of (CAG)140 (A) Non-selective growth on galactose. Selection at 60 μg/mL canavanine concentration (B) Non-selective growth on glucose. Selection at 60 μg/mL canavanine concentration Dashed line designates 3-fold decrease from wild-type. Numbers above the dashed line show fold decrease compared to wild-type. Dotted line in (A) indicates 3-fold increase from wild-type.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4 and Supplementary Tables 1–4 (PDF 1023 kb)
Rights and permissions
About this article
Cite this article
Kim, J., Harris, S., Dinter, T. et al. The role of break-induced replication in large-scale expansions of (CAG)n/(CTG)n repeats. Nat Struct Mol Biol 24, 55–60 (2017). https://doi.org/10.1038/nsmb.3334
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.3334
This article is cited by
-
Structure-forming CAG/CTG repeats interfere with gap repair to cause repeat expansions and chromosome breaks
Nature Communications (2023)
-
Group II intron and repeat-rich red algal mitochondrial genomes demonstrate the dynamic recent history of autocatalytic RNAs
BMC Biology (2022)
-
Conformational and migrational dynamics of slipped-strand DNA three-way junctions containing trinucleotide repeats
Nature Communications (2021)
-
Trinucleotide repeat instability during double-strand break repair: from mechanisms to gene therapy
Current Genetics (2019)
-
Mechanisms of genetic instability caused by (CGG)n repeats in an experimental mammalian system
Nature Structural & Molecular Biology (2018)