Abstract
Noncoding RNAs, mobile elements, and alternative splicing are all critical for the regulation of gene expression. Here we show that a conserved noncoding RNA acquires a new function due to the insertion of a mobile element. We identified a noncoding RNA, termed 5S-OT, which is transcribed from 5S rDNA loci in eukaryotes including fission yeast and mammals. 5S-OT plays a cis role in regulating the transcription of 5S rRNA in mice and humans. In the anthropoidea suborder of primates, an antisense Alu element has been inserted at the 5S-OT locus. We found that in human cells, 5S-OT regulates alternative splicing of multiple genes in trans via Alu/anti-Alu pairing with target genes and by interacting with the splicing factor U2AF65. This trans effect of 5S-OT in splicing might be exploited in biotechnological applications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Quinn, J.J. & Chang, H.Y. Unique features of long non-coding RNA biogenesis and function. Nat. Rev. Genet. 17, 47–62 (2016).
Cech, T.R. & Steitz, J.A. The noncoding RNA revolution-trashing old rules to forge new ones. Cell 157, 77–94 (2014).
Guttman, M. & Rinn, J.L. Modular regulatory principles of large non-coding RNAs. Nature 482, 339–346 (2012).
Bogdanov, A.A., Dontsova, O.A., Dokudovskaya, S.S. & Lavrik, I.N. Structure and function of 5S rRNA in the ribosome. Biochem. Cell Biol. 73, 869–876 (1995).
Ciganda, M. & Williams, N. Eukaryotic 5S rRNA biogenesis. Wiley Interdiscip. Rev. RNA 2, 523–533 (2011).
Haeusler, R.A. & Engelke, D.R. Spatial organization of transcription by RNA polymerase III. Nucleic Acids Res. 34, 4826–4836 (2006).
Paule, M.R. & White, R.J. Survey and summary: transcription by RNA polymerases I and III. Nucleic Acids Res. 28, 1283–1298 (2000).
Hu, S., Wu, J., Chen, L. & Shan, G. Signals from noncoding RNAs: unconventional roles for conventional pol III transcripts. Int. J. Biochem. Cell Biol. 44, 1847–1851 (2012).
Oler, A.J. et al. Human RNA polymerase III transcriptomes and relationships to Pol II promoter chromatin and enhancer-binding factors. Nat. Struct. Mol. Biol. 17, 620–628 (2010).
Barski, A. et al. Pol II and its associated epigenetic marks are present at Pol III–transcribed noncoding RNA genes. Nat. Struct. Mol. Biol. 17, 629–634 (2010).
Tata, J.R., Hamilton, M.J. & Shields, D. Effects of α-amanitin in vivo on RNA polymerase and nuclear RNA synthesis. Nat. New Biol. 238, 161–164 (1972).
Wang, L. et al. CPAT: Coding-Potential Assessment Tool using an alignment-free logistic regression model. Nucleic Acids Res. 41, e74 (2013).
Chu, C., Qu, K., Zhong, F.L., Artandi, S.E. & Chang, H.Y. Genomic maps of long noncoding RNA occupancy reveal principles of RNA-chromatin interactions. Mol. Cell 44, 667–678 (2011).
Batzer, M.A. & Deininger, P.L. Alu repeats and human genomic diversity. Nat. Rev. Genet. 3, 370–379 (2002).
Ruskin, B., Zamore, P.D. & Green, M.R. A factor, U2AF, is required for U2 snRNP binding and splicing complex assembly. Cell 52, 207–219 (1988).
Deininger, P. Alu elements: know the SINEs. Genome Biol. 12, 236 (2011).
Gooding, C., Kemp, P. & Smith, C.W. A novel polypyrimidine tract-binding protein paralog expressed in smooth muscle cells. J. Biol. Chem. 278, 15201–15207 (2003).
Shao, C. et al. Mechanisms for U2AF to define 3′ splice sites and regulate alternative splicing in the human genome. Nat. Struct. Mol. Biol. 21, 997–1005 (2014).
Zarnack, K. et al. Direct competition between hnRNP C and U2AF65 protects the transcriptome from the exonization of Alu elements. Cell 152, 453–466 (2013).
Auwerx, J. The human leukemia cell line, THP-1: a multifacetted model for the study of monocyte-macrophage differentiation. Experientia 47, 22–31 (1991).
Tripathi, V. et al. The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol. Cell 39, 925–938 (2010).
Gonzalez, I. et al. A lncRNA regulates alternative splicing via establishment of a splicing-specific chromatin signature. Nat. Struct. Mol. Biol. 22, 370–376 (2015).
Skordis, L.A., Dunckley, M.G., Yue, B., Eperon, I.C. & Muntoni, F. Bifunctional antisense oligonucleotides provide a trans-acting splicing enhancer that stimulates SMN2 gene expression in patient fibroblasts. Proc. Natl. Acad. Sci. USA 100, 4114–4119 (2003).
Deaton, A.M. & Bird, A. CpG islands and the regulation of transcription. Genes Dev. 25, 1010–1022 (2011).
Marcovitz, A., Jia, R. & Bejerano, G. “Reverse genomics” predicts function of human conserved non-coding elements. Mol. Biol. Evol. 33, 1358–1369 (2016).
Li, M. & Gu, W. A critical role for noncoding 5S rRNA in regulating Mdmx stability. Mol. Cell 43, 1023–1032 (2011).
Listerman, I., Bledau, A.S., Grishina, I. & Neugebauer, K.M. Extragenic accumulation of RNA polymerase II enhances transcription by RNA polymerase III. PLoS Genet. 3, e212 (2007).
White, R.J. Transcription by RNA polymerase III: more complex than we thought. Nat. Rev. Genet. 12, 459–463 (2011).
Ule, J. Alu elements: at the crossroads between disease and evolution. Biochem. Soc. Trans. 41, 1532–1535 (2013).
Jeck, W.R. et al. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA 19, 141–157 (2013).
Li, Z. et al. Exon-intron circular RNAs regulate transcription in the nucleus. Nat. Struct. Mol. Biol. 22, 256–264 (2015).
Konkel, M.K., Walker, J.A. & Batzer, M.A. LINEs and SINEs of primate evolution. Evol. Anthropol. 19, 236–249 (2010).
Denli, A.M. et al. Primate-specific ORF0 contributes to retrotransposon-mediated diversity. Cell 163, 583–593 (2015).
Gueroussov, S. et al. An alternative splicing event amplifies evolutionary differences between vertebrates. Science 349, 868–873 (2015).
Necsulea, A. et al. The evolution of lncRNA repertoires and expression patterns in tetrapods. Nature 505, 635–640 (2014).
Diederichs, S. The four dimensions of noncoding RNA conservation. Trends Genet. 30, 121–123 (2014).
Morris, K.V. & Mattick, J.S. The rise of regulatory RNA. Nat. Rev. Genet. 15, 423–437 (2014).
Shan, G. RNA interference as a gene knockdown technique. Int. J. Biochem. Cell Biol. 42, 1243–1251 (2010).
Dubin, R.A., Kazmi, M.A. & Ostrer, H. Inverted repeats are necessary for circularization of the mouse testis Sry transcript. Gene 167, 245–248 (1995).
Carey, M.F., Peterson, C.L. & Smale, S.T. The RNase Protection Assay. Cold Spring Harb. Protoc. 2013, pdb.prot071910 (2013).
Maamar, H., Cabili, M.N., Rinn, J. & Raj, A. linc-HOXA1 is a noncoding RNA that represses Hoxa1 transcription in cis. Genes Dev. 27, 1260–1271 (2013).
Kent, W.J. et al. The human genome browser at UCSC. Genome Res. 12, 996–1006 (2002).
Yeo, G. & Burge, C.B. Maximum entropy modeling of short sequence motifs with applications to RNA splicing signals. J. Comput. Biol. 11, 377–394 (2004).
Gardiner-Garden, M. & Frommer, M. CpG islands in vertebrate genomes. J. Mol. Biol. 196, 261–282 (1987).
Acknowledgements
We thank X. Wang (HUST), J. Liu (HFUT), and Y. Zhou (Wuhan University) for reagents. We thank L. Chen and other members of the Shan laboratory for discussions and technical support. This work was supported by the National Basic Research Program of China (2015CB943000 to G.S.), the National Natural Science Foundation of China (91519333 and 31471225 to G.S.), the CAS Key Laboratory of Innate Immunity and Chronic Disease (G.S.), and the Fundamental Research Funds for the Central Universities (WK2070000034 to G.S.).
Author information
Authors and Affiliations
Contributions
G.S. conceived the project and supervised its execution. G.S., S.H., and X.W. designed experiments, analyzed data, and wrote the manuscript. S.H. and X.W. performed the experiments. X.W. performed bioinformatic analyses. All authors discussed the results and made comments on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
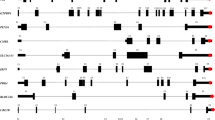
Supplementary Figure 1 Characterization of mammalian 5S-OT as a pol II transcript.
a, Strand specific RT-PCR of 5S-OT in human and mice. SSP-F, RT primer with sense direction to 5S rRNA. SSP-R, RT primer with antisense direction to 5S rRNA.
b, RT-PCR of h5S-OT in different tissues and cell lines. Actin was shown as a positive control.
c, RT-PCR of m5S-OT in different tissues and cell lines. Actin was shown as a positive control.
d, Real-time PCR showing the decrease of 5S-OT level after 24h α-amanitin treatment in cells of human (Hela and 293T) and mice (N2a and 3T3). GAPDH mRNA, positive control; 18S rRNA (a pol I transcript), negative control.
e, ChIP showing binding of pol II to the promoter and gene body of h5S-OT (Hela cells) and m5S-OT (N2a cells). Actin as negative controls in western blots; 18S promoter as negative controls in Real-time PCR. The pattern of pol II binding for h5S-OT and m5S-OT has a peak at the first nucleosome from the TSS (~ 200 bp downstream), a feature of some “paused, expressed” genes in metazoans (Adelman K & Lis JT, 2012).
f, Cumulative probability distribution of coding potential as measured by CPAT for both noncoding transcripts and coding transcripts12. Hsa-5S rRNA and mus-5S rRNA are negative controls, and hsa-GAPDH and mus-GAPDH are positive controls.
Error bars, s.e.m. from triplicate experiments. N. S., not significant; *P < 0.05; **P < 0.01; ***P < 0.001 by two-tailed Student’s t test.
Adelman, K. & Lis, J. T. Promoter-proximal pausing of RNA polymerase II: emerging roles in metazoans. Nat Rev Genet 13, 720-31 (2012).
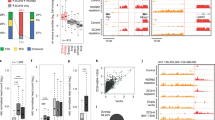
Supplementary Figure 2 Cis effect of mammalian 5S-OT.
a, Nuclear run-on assays showing the decrease of 5S and 5S-OT in transcription after 24h α-amanitin treatment at a concentration of 0.5 μg/ml and 1.0 μg/ml in 293T and 3T3 respectively. 18S rRNA (a pol I transcript), negative control.
b, Knocking down 5S-OT with siRNAs in human (Hela and 293T) or mice (N2a and 3T3) cells did not affect the total levels of 5S rRNA.
c, Nuclear run-on assays showing the decrease in the transcription of 5S but not the 5S-OT after 5S-OT knockdown using two individual siRNAs in 293T and 3T3, respectively. NC, siRNAs with scrambled sequences. U6 snRNA, another pol III transcript, negative control for 5S rRNA.
d, Knocking down 5S-OT in human (Hela and 293T) cells with ASO did not affect the total levels of 5S rRNA, but decreased the transcription of 5S but not the 5S-OT. NC-ASO, ASO with scrambled sequences. U6 snRNA, another pol III transcript, negative control for 5S rRNA.
e, Efficiency of RNA pulldown in ChIRP shown in Fig. 1f.
Error bars, s.e.m. from triplicate experiments. *P < 0.05; **P < 0.01; ***P < 0.001 by two-tailed Student’s t test.
Supplementary Figure 3 h5S-OT knockdown leads to changes in alternative splicing.
a, Percentage of inclusion or exclusion (p<0.05) of h5S-OT sensitive exons in Hela and 293T cells.
b, Overlapped h5S-OT sensitive exons in 293T and Hela cells.
c, RT-PCR validation of 5S-OT sensitive exons in 293T and Hela cells with or without siRNA / ASO knocking down. For the RT-PCR gel of NUMB, an unspecific band was also amplified (indicated with a triangle). For KIAA1191 and NOSIP, western blots were performed to examine the protein levels of the corresponding isoforms. NC, negative control with scrambled sequences.
Supplementary Figure 4 Comparison of effects of h5S-OT and m5S-OT on gene expression and alternative splicing, 5S-OT copy numbers, and possible indirect effects of h5S-OT in alternative splicing.
a, Heatmaps of gene expression (≥two fold change and p<0.01, genes with RPKM≥1 counted as expression) upon h5S-OT knockdown in 293T and Hela cells. siRNAs with scrambled sequences were used as negative control.
b, Boolean distribution of cassette exon in genes showed significant changes in expression levels upon h5S-OT knockdown. Control, 300 randomly chosen genes. Without cassette exon (0), with cassette exon (1).
c, Plot of gene expression upon m5S-OT knockdown in murine N2a cells.
d, Number of exons showed significant (p<0.01) changes in alternative splicing upon knocking down of 5S-OT in human (293T and Hela) and murine N2a cells. Total number of cassette exons detected in each cell line is indicated.
e, Plot of 5S-OT copy numbers in human and mice cell lines.
f, Boolean distribution of sense Alu sequences in full length pre-mRNA of h5S-OT sensitive genes. Control, all genes with cassette exons detected in 293T and Hela. No sense Alu sequences (0), with sense Alu sequences (1).
g, Splicing factor genes sensitive to h5S-OT. Overlaps between h5S-OT sensitive genes and PTBP3 RIP data (Brazão, et al., 2012) from 293T cells are shown. Isoform specific knocking down of PTBP3 affected the splicing of PUM2 (One of the 31 overlapped genes). Interestingly, there is no sense Alu within 2 knt upstream or downstream of the cassette exon in PUM2.
Brazão, T. F. et al. A new function of ROD1 in nonsense-mediated mRNA decay. FEBS Lett 586, 1101-10 (2012).
Supplementary Figure 5 Examination of the h5S-OT trans effect, interacting proteins, the Py site, and interaction between 5S-OT and splicing factors.
a, Mass spectrometry result of the h5S-OT specific band shown in Fig. 4a.
b, G3BP-2a and h5S-OT has no interaction with each other. RIP with FLAG antibody for FLAG-G3BP-2a did not pulldown h5S-OT; h5S-OT RNA pulldown did not co-pulldown G3BP-2a (U2AF65, positive control; Actin, negative control).
c, Alignment of Py site in the 5S-OT of Human, Chimp, Gorilla, Orangutan and Green monkey.
d, PTBP1 has no interaction with h5S-OT. RIP with FLAG antibody targeting the overexpressed FLAG-PTBP1 did not pull down h5S-OT. GAPDH mRNA was a negative controls, and U1 snRNA was a positive control.
e, Pulldown of U2AF65 protein did not pulldown m5S-OT. Western blots showing efficient pulldown of U2AF65 with Actin as a negative control. U2 snRNA, known to interact with U2AF65, was a positive control for m5S-OT, and U7 snRNA as a negative control.
f, Pulldown of U2AF65 protein did not pulldown m5S-OT even when it was overexpressed. Western blots showing efficient pulldown of U2AF65 with Actin as a negative control. U2 snRNA, known to interact with U2AF65, was a positive control for m5S-OT, and U7 snRNA as a negative control.
g, Pulldown of U2AF65 protein co-pulled down h5S-OT when it was artificially expressed in murine cells. Western blots showing efficient pulldown of U2AF65 with Actin as a negative control. U2 snRNA, known to interact with U2AF65, was a positive control, and U7 snRNA as a negative control.
Error bars, s.e.m. from triplicate experiments. *P < 0.05; **P < 0.01; ***P < 0.001 by two-tailed Student’s t test.
Supplementary Figure 6 U2AF65-sensitive exons and h5S-OT/U2AF65 and h5S-OT/PTBP1 relationships.
a, Heatmaps of ΔPSI upon knockdown of U2AF65 in 293T and Hela cells with two independent siRNAs. siRNAs with scrambled sequences were used as negative control. Cassette exons with significant (P <0.01) changes in PSI are shown. P values were generated by two-tailed Mann-Whitney U test.
b, Correlation plot of ΔPSI from two independent siRNAs in human 293T and Hela cells, respectively.
c, Venn diagrams showing the overlap of U2AF65 sensitive exons in Hela cells between our data and GSE6160318. We counted exons with significant (P<0.01) changes in ΔPSI, whereas the Ref. 16 also applied a cutoff of ΔPSI≥0.15 besides P<0.01.
d, Real-time PCR showing that knocking down either h5S-OT or U2AF65 did not change the expression level of the other.
e, Western blots showing that protein level of U2AF65 did not change when knocking down h5S-OT. Actin, loading control. NC, negative control with scrambled sequences.
f, Venn diagram demonstrating the overlap between h5S-OT and PTBP1 sensitive exons in human 293T. Knocking down efficiency of PTBP1 is shown in the bar figure. Vector (shCtr) of the shRNA construct was used as a control.
Error bars, s.e.m. from triplicate experiments. *P < 0.05; **P < 0.01; ***P < 0.001 by two-tailed Student’s t test.
Supplementary Figure 7 Validation of h5S-OT- and U2AF65-sensitive exons and RIP efficiency of U2AF65.
a, RT-PCR validation of h5S-OT and U2AF65 sensitive exons in 293T and Hela cells.
b, Overexpression of U2AF65 did not compensate for h5S-OT knockdown in the effect of alternative splicing in two examples examined with 293T cells.
c, Pulldown of h5S-OT in RIP with an antibody against U2AF65 with or without the knocking down of h5S-OT. Western blots showing efficient pulldown of U2AF65 with Actin as a negative control. U2 snRNA, known to interact with U2AF65, was a positive control for h5S-OT, and U7 snRNA as a negative control.
d, Pulldown of h5S-OT in RIP with an antibody against U2AF65 upon the overexpression of h5S-OT. h5S-OT without the antisense Alu (ΔAlu) was used as a comparison. Western blots showing efficient pulldown of U2AF65 with Actin as a negative control. U2 snRNA, the positive control; U7 snRNA, the negative control.
Error bars, s.e.m. from triplicate experiments. *P < 0.05; ***P < 0.001 by two-tailed Student’s t test.
Supplementary Figure 8 Examples of gene-specific h5S-OT that did not affect alternative splicing, 5S-OT structure in model organisms, the h5S-OT/m5S-OT promoter, and the strength of flanking 3’ SSs of h5S-OT- and U2AF65-sensitive exons.
a, Examples of “gene specific” h5S-OT failed in affecting the alternative splicing. Target upstream (5’) 3’SS or downstream (3’) 3’SS with “gene specific” h5S-OT did not change the splicing pattern of the corresponding cassette exon.
b, Human and mouse 5S-OT structure, 5S rDNA region of model organisms, and annotation of repeats.
c, CpG island in the promoter proximity of h5S-OT and m5S-OT. No TATA box is present in either promoter. With the results shown in Sfig. 1e, it seems that both h5S-OT and m5S-OT promoters are “broad peak promoter”, which often associates with ubiquitously expressed genes (Müller, et al., 2007). Shown below are EGR2 binding sites in the promoter of h5S-OT detected with MEME tool. There is no extra transcription factor binding site in the m5S-OT promoter region as against h5S-OT promoter.
d, Both h5S-OT and U2AF65 sensitive exons tend to have a relatively stronger 3’ SS for their immediate downstream introns than the 3’ SS of their immediate upstream introns. Control, 300 randomly chosen human cassette exons. N. S., not significant; **, P<0.01; ***, P<0.001; P values were generated by two-tailed Mann-Whitney U test.
Müller, F., Demény, M. A. & Tora, L. New problems in RNA polymerase II transcription initiation: matching the diversity of core promoters with a variety of promoter recognition factors. J Biol Chem 282, 14685-14689 (2007).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 and Supplementary Table 1 (PDF 2795 kb)
Supplementary Data Set 1
Uncropped images (PDF 328 kb)
Rights and permissions
About this article
Cite this article
Hu, S., Wang, X. & Shan, G. Insertion of an Alu element in a lncRNA leads to primate-specific modulation of alternative splicing. Nat Struct Mol Biol 23, 1011–1019 (2016). https://doi.org/10.1038/nsmb.3302
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.3302
This article is cited by
-
MGCG regulates glioblastoma tumorigenicity via hnRNPK/ATG2A and promotes autophagy
Cell Death & Disease (2023)
-
The long non-coding RNA DKFZp434J0226 regulates the alternative splicing process through phosphorylation of SF3B6 in PDAC
Molecular Medicine (2021)
-
Genome-wide analysis of long noncoding RNA expression profile in nasal mucosa with allergic rhinitis
BMC Medical Genomics (2021)
-
Host cell response and distinct gene expression profiles at different stages of Chlamydia trachomatis infection reveals stage-specific biomarkers of infection
BMC Microbiology (2021)
-
Long non-coding RNA LPP-AS2 promotes glioma tumorigenesis via miR-7-5p/EGFR/PI3K/AKT/c-MYC feedback loop
Journal of Experimental & Clinical Cancer Research (2020)