Abstract
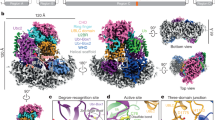
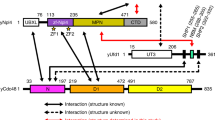
Pan2–Pan3 is a conserved complex involved in the shortening of mRNA poly(A) tails, the initial step in eukaryotic mRNA turnover. We show that recombinant Saccharomyces cerevisiae Pan2–Pan3 can deadenylate RNAs in vitro without needing the poly(A)-binding protein Pab1. The crystal structure of an active ~200-kDa core complex reveals that Pan2 and Pan3 interact with an unusual 1:2 stoichiometry imparted by the asymmetric nature of the Pan3 homodimer. An extended region of Pan2 wraps around Pan3 and provides a major anchoring point for complex assembly. A Pan2 module formed by the pseudoubiquitin-hydrolase and RNase domains latches onto the Pan3 pseudokinase with intertwined interactions that orient the deadenylase active site toward the A-binding site of the interacting Pan3. The molecular architecture of Pan2–Pan3 suggests how the nuclease and its pseudokinase regulator act in synergy to promote deadenylation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Subtelny, A.O., Eichhorn, S.W., Chen, G.R., Sive, H. & Bartel, D.P. Poly(A)-tail profiling reveals an embryonic switch in translational control. Nature 508, 66–71 (2014).
Goss, D.J. & Kleiman, F.E. Poly(A) binding proteins: are they all created equal? Wiley Interdiscip. Rev. RNA 4, 167–179 (2013).
Kapp, L.D. & Lorsch, J.R. The molecular mechanics of eukaryotic translation. Annu. Rev. Biochem. 73, 657–704 (2004).
Bernstein, P., Peltz, S.W. & Ross, J. The poly(A)-poly(A)-binding protein complex is a major determinant of mRNA stability in vitro. Mol. Cell. Biol. 9, 659–670 (1989).
Brook, M. & Gray, N.K. The role of mammalian poly(A)-binding proteins in co-ordinating mRNA turnover. Biochem. Soc. Trans. 40, 856–864 (2012).
Decker, C.J. & Parker, R. A turnover pathway for both stable and unstable mRNAs in yeast: evidence for a requirement for deadenylation. Genes Dev. 7, 1632–1643 (1993).
Brown, C.E. & Sachs, A.B. Poly(A) tail length control in Saccharomyces cerevisiae occurs by message-specific deadenylation. Mol. Cell. Biol. 18, 6548–6559 (1998).
Chen, C.-Y.A. & Shyu, A.-B. Mechanisms of deadenylation-dependent decay. Wiley Interdiscip. Rev. RNA 2, 167–183 (2011).
Wahle, E. & Winkler, G.S. RNA decay machines: deadenylation by the Ccr4-Not and Pan2-Pan3 complexes. Biochim. Biophys. Acta 1829, 561–570 (2013).
Parker, R. & Song, H. The enzymes and control of eukaryotic mRNA turnover. Nat. Struct. Mol. Biol. 11, 121–127 (2004).
Garneau, N.L., Wilusz, J. & Wilusz, C.J. The highways and byways of mRNA decay. Nat. Rev. Mol. Cell Biol. 8, 113–126 (2007).
Chekulaeva, M. et al. miRNA repression involves GW182-mediated recruitment of CCR4–NOT through conserved W-containing motifs. Nat. Struct. Mol. Biol. 18, 1218–1226 (2011).
Braun, J.E., Huntzinger, E., Fauser, M. & Izaurralde, E. GW182 proteins directly recruit cytoplasmic deadenylase complexes to miRNA targets. Mol. Cell 44, 120–133 (2011).
Fabian, M.R. et al. miRNA-mediated deadenylation is orchestrated by GW182 through two conserved motifs that interact with CCR4–NOT. Nat. Struct. Mol. Biol. 18, 1211–1217 (2011).
Barckmann, B. & Simonelig, M. Control of maternal mRNA stability in germ cells and early embryos. Biochim. Biophys. Acta 1829, 714–724 (2013).
Yamashita, A. et al. Concerted action of poly(A) nucleases and decapping enzyme in mammalian mRNA turnover. Nat. Struct. Mol. Biol. 12, 1054–1063 (2005).
Boeck, R. et al. The yeast Pan2 protein is required for poly(A)-binding protein-stimulated poly(A)-nuclease activity. J. Biol. Chem. 271, 432–438 (1996).
Tucker, M., Staples, R.R., Valencia-Sanchez, M.A., Muhlrad, D. & Parker, R. Ccr4p is the catalytic subunit of a Ccr4p/Pop2p/Notp mRNA deadenylase complex in Saccharomyces cerevisiae. EMBO J. 21, 1427–1436 (2002).
Sun, M. et al. Global analysis of eukaryotic mRNA degradation reveals Xrn1-dependent buffering of transcript levels. Mol. Cell 52, 52–62 (2013).
Maillet, L. & Collart, M.A. Interaction between Not1p, a component of the Ccr4-not complex, a global regulator of transcription, and Dhh1p, a putative RNA helicase. J. Biol. Chem. 277, 2835–2842 (2002).
Basquin, J. et al. Architecture of the nuclease module of the yeast Ccr4-Not complex: the Not1-Caf1-Ccr4 interaction. Mol. Cell 48, 207–218 (2012).
Petit, A.-P. et al. The structural basis for the interaction between the CAF1 nuclease and the NOT1 scaffold of the human CCR4-NOT deadenylase complex. Nucleic Acids Res. 40, 11058–11072 (2012).
Fabian, M.R. et al. Structural basis for the recruitment of the human CCR4–NOT deadenylase complex by tristetraprolin. Nat. Struct. Mol. Biol. 20, 735–739 (2013).
Bhaskar, V. et al. Structure and RNA-binding properties of the Not1–Not2–Not5 module of the yeast Ccr4–Not complex. Nat. Struct. Mol. Biol. 20, 1281–1288 (2013).
Boland, A. et al. Structure and assembly of the NOT module of the human CCR4–NOT complex. Nat. Struct. Mol. Biol. 20, 1289–1297 (2013).
Brown, C.E., Tarun, S.Z., Boeck, R. & Sachs, A.B. PAN3 encodes a subunit of the Pab1p-dependent poly(A) nuclease in Saccharomyces cerevisiae. Mol. Cell. Biol. 16, 5744–5753 (1996).
Mangus, D.A. et al. Positive and negative regulation of poly(A) nuclease. Mol. Cell. Biol. 24, 5521–5533 (2004).
Christie, M., Boland, A., Huntzinger, E., Weichenrieder, O. & Izaurralde, E. Structure of the PAN3 pseudokinase reveals the basis for interactions with the PAN2 deadenylase and the GW182 proteins. Mol. Cell 51, 360–373 (2013).
Zuo, Y. & Deutscher, M.P. Exoribonuclease superfamilies: structural analysis and phylogenetic distribution. Nucleic Acids Res. 29, 1017–1026 (2001).
Siddiqui, N. et al. Poly(A) nuclease interacts with the C-terminal domain of polyadenylate-binding protein domain from poly(A)-binding protein. J. Biol. Chem. 282, 25067–25075 (2007).
Lowell, J.E., Rudner, D.Z. & Sachs, A.B. 3′-UTR-dependent deadenylation by the yeast poly(A) nuclease. Genes Dev. 6, 2088–2099 (1992).
Uchida, N., Hoshino, S.-I. & Katada, T. Identification of a human cytoplasmic poly(A) nuclease complex stimulated by poly(A)-binding protein. J. Biol. Chem. 279, 1383–1391 (2004).
Horio, T. et al. Crystal structure of human ISG20, an interferon-induced antiviral ribonuclease. FEBS Lett. 577, 111–116 (2004).
Hu, M. et al. Crystal structure of a UBP-family deubiquitinating enzyme in isolation and in complex with ubiquitin aldehyde. Cell 111, 1041–1054 (2002).
Holm, L. & Rosenström, P. Dali server: conservation mapping in 3D. Nucleic Acids Res. 38, W545–W549 (2010).
Thore, S., Mauxion, F., Seraphin, B. & Suck, D. X-ray structure and activity of the yeast Pop2 protein: a nuclease subunit of the mRNA deadenylase complex. EMBO Rep. 4, 1150–1155 (2003).
Wang, H. et al. Crystal structure of the human CNOT6L nuclease domain reveals strict poly(A) substrate specificity. EMBO J. 29, 2566–2576 (2010).
Bayliss, R., Sardon, T., Vernos, I. & Conti, E. Structural basis of Aurora-A activation by TPX2 at the mitotic spindle. Mol. Cell 12, 851–862 (2003).
Sachs, A.B. & Deardorff, J.A. Translation initiation requires the PAB-dependent poly(A) ribonuclease in yeast. Cell 70, 961–973 (1992).
Huang, H. et al. Dimeric structure of pseudokinase RNase L bound to 2-5A reveals a basis for interferon-induced antiviral activity. Mol. Cell 53, 221–234 (2014).
Weiss, M.S. Global indicators of X-ray data quality. J. Appl. Crystallogr. 34, 130–135 (2001).
Evans, P.R. & Murshudov, G.N. How good are my data and what is the resolution? Acta Crystallogr. D Biol. Crystallogr. 69, 1204–1214 (2013).
Diederichs, K. & Karplus, P.A. Better models by discarding data? Acta Crystallogr. D Biol. Crystallogr. 69, 1215–1222 (2013).
Fitzgerald, D.J. et al. Protein complex expression by using multigene baculoviral vectors. Nat. Methods 3, 1021–1032 (2006).
Halbach, F., Reichelt, P., Rode, M. & Conti, E. The yeast ski complex: crystal structure and RNA channeling to the exosome complex. Cell 154, 814–826 (2013).
Kabsch, W. Integration, scaling, space-group assignment and post-refinement. Acta Crystallogr. D Biol. Crystallogr. 66, 133–144 (2010).
Adams, P.D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Emsley, P., Lohkamp, B., Scott, W.G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Afonine, P.V. et al. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr. D Biol. Crystallogr. 68, 352–367 (2012).
McCoy, A.J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Read, R.J. & McCoy, A.J. Using SAD data in Phaser. Acta Crystallogr. D Biol. Crystallogr. 67, 338–344 (2011).
Chen, V.B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 66, 12–21 (2010).
Acknowledgements
We would like to thank A. Fischer for data in Figure 5b and C. Basquin for Thermofluor experiments (both Max Planck Institute (MPI) of Biochemistry); A. McCoy (Cambridge Institute for Medical Research) for advice on Phaser; the MPI Crystallization Facility for crystal screening and optimization; the MPI Core Facility for MS; and the beamline scientists at the Swiss Light Source (SLS) for excellent assistance with data collection. We also thank members of our laboratory for useful discussions and critical reading of the manuscript. This study was supported by the Max Planck Gesellschaft and grants of the European Research Council (ERC Advanced Investigator Grant 294371 and Marie Curie Initial Training Networks RNPnet) and the Deutsche Forschungsgemeinschaft (DFG SFB646, SFB1035, GRK1721, FOR1680 and CIPSM) to E.C.
Author information
Authors and Affiliations
Contributions
I.B.S. performed molecular biology and biochemical analysis and determined structures. M.R. assisted with insect-cell culture. F.B. performed deadenylation assays. S.S. helped with protein purifications. I.B.S. and E.C. designed the study and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 The WD40 domain of Pan2 directly contacts the Pan2–Pan3 core complex.
(a) RNase assays with Pan2fl–Pan3fl-H10 using different 15mer homo-oligonucleotides, carried out as in Fig. 1c. Abbreviation: conc. (concentration). (b) Protein co-precipitation of H10Pan3CR (His-tagged Pan3 C-terminal region, residues 275–679) and Pan2fl (Pan2 full-length). The Nickel pull-down assay was performed on the cell lysate in the presence of 300 mM salt and samples were separated on 4-12% SDS-PAGE. (c) Left panel: protein co-precipitation of Pan3ΔN-H10 (His tagged Pan3 residues 226–679) and Pan2N-3C-C (fl Pan2 with a 3C protease cleavage site engineered after the N-terminal WD40 domain, between residues 333 and 334). The Nickel pull-down assay (His-Select resin, Sigma) was carried as described in panel a. Central panel: analysis of the Pan3ΔN-H10– Pan2N-3C-C sample after 3C protease cleavage (lane 1). The cleaved sample was purified on an ion exchange column (AEC), which separated the excess of 3C protease (lanes 2-4) from three polypeptides which co-eluted in the same peak: Pan2N (residues 1–333), Pan2C (residues 334–1115) and Pan3ΔN-H10 (lane 5). The three peptides also co-eluted by size exclusion chromatography (SEC,lane 9). Right panel: chromatogram of the size-exclusion chromatography. Highlighted in red is the peak fraction corresponding to lane 9 of the gel on the left. Abbreviations: 3C-prot. (3C-protease), A280nm (Absorption at 280 nm).
Supplementary Figure 2 Structural and biochemical analysis of Pan2–Pan3.
(a) Snapshots of the simulated annealing Fo-Fc omit map countered at 2.5σ (mesh, in grey) for different segments of the Pan2 linker. The anomalous signal peak for the Selenomethionine residues is shown in red, contoured at 4.0σ. Abbreviation: UCH (ubiquitin C-terminal hydrolase). (b) Size exclusion chromatography profile of Pan2fl–Pan3ΔN with standard molecular weight markers. The calculated mass of the complex is shown on the right. Abbreviations: Pan3ΔN (residues 226–679), H10 (His-tag), fl (full length), MW (molecular weight), A280nm (Absorption at 280 nm).
Supplementary Figure 3 Conserved interactions centered on Pan3.
(a) Superposition of the atomic models of the individual protomers of a Pan3ΔN dimer from the yeast Pan2ΔN–Pan3ΔN structure (with the two chains in yellow and orange) with those of the N. crassa Pan3CR dimer28 (in gray, left panel) and of the D. melanogaster (in gray, right pane) in isolation. Note that helix αN (from the extension upstream of the pseudokinase domain) is not present in the D. melanogaster structure. Abbreviation: CR (C-terminal region, residues 275–679). (b) The surfaces of the knob domains are shown, colored according to sequence conservation from light (less conserved) to dark (conserved), with the Pan2 linker in ribbon representation. Indicated are the positions of the residues of yeast Pan3 corresponding to mutants previously described28. Abbreviation: N-term. (N-terminus).
Supplementary Figure 4 Comparison of Pan2ΔN–Pan3ΔN domains with related domains in other proteins.
(a) The UCH domain of Pan2 (left) is viewed in the same orientation as the UCH domain of HAUSP34, after optimal superposition. Both domains are colored with the Fingers in cyan, the Palm in blue and the Thumb in green. The active site of HAUSP and the equivalent position Pan2 CH are indicated with an asterisk. The active-site residues of HAUSP domain are shown in stick representation. The residues at the equivalent position of the inactive Pan2 UCH are also shown. Abbreviations: Pan2R (RNase domain of Pan2), Pan2U (ubiquitin C-terminal hydrolase of Pan2), UCH (ubiquitin C-terminal hydrolase). (b) The RNase domain of Pan2 (left panel) is viewed in the same orientation as the similar domains in the ISG20 RNase33 (central panel) and in yeast Caf1 (also known as Pop2)36 (right panel) after optimal superposition. Active site residues are highlighted in stick representation. The nucleotides in the active sites are also shown in stick representation, together with the ions as spheres. (c) The overall structure UCH–RNase module of Pan2 is essentially unchanged when in the Pan2ΔN–Pan3ΔN complex (UCH in blue, cyan and green, and RNase in pink) and in the structure of Pan2UR in isolation (in gray), with the exception of a loop that becomes ordered in the complex (corresponding to the loop shown in Supplementary Fig. 4d and in stick representation in Fig. 5a). (d) The N-terminal lobe of the Pan3bent pseudokinase (yellow) interacts with a loop of the Pan2 RNase domain (left and central panel) at a similar overall position used for interaction in either kinases, for example between Aurora-A (orange) and TPX2 (red)38 (right panel, shown in the same orientation after optimal superposition of the N-terminal lobes).
Supplementary Figure 5 Characterization of the Pan2–Pan3–nucleotide interactions.
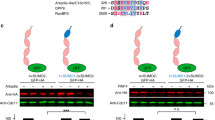
(a) Thermofluor assay comparing the stability of Pan2ΔN–Pan3ΔN-ATP and Pan2ΔN–Pan3ΔN used in the assay in Fig. 6b. The curves show the thermal unfolding transition. The proteins were incubated with 3.5X of Sypro Orange dye (Invitrogen), which binds to hydrophobic patches exposed on the protein during unfolding). The proteins were subjected to thermal denaturation and the change in fluorescence intensity was measured with a real-time PCR system (Eppendorf). The apparent melting temperatures of Pan2ΔN–Pan3ΔN and Pan2ΔN–Pan3ΔN-ATP mutant measurements with the Thermofluor assay in the presence and absence of AMP are shown. Abbreviations: Pan2ΔN (residues 340–1115), Pan3ΔN (residues 226– 679), Pan3ATP (ATP binding mutant of Pan3). (b) Deadenylation assays using the protein samples shown in Fig. 6c in the absence (left panels) and presence of 1mM ATP. Abbreviations: M (size markers), conc. (concentration).
Supplementary Figure 6 Interaction sites in the Pan2–Pan3 complex.
Structure of the yeast Pan2ΔN–Pan3ΔN complex with the bound AMP molecule at the deadenylase active site. Also shown are the nucleotides (ATPγS) and the region corresponding to the Tryptophan-binding pocket from the structures of Pan3 orthologues28, after optimal superposition. Abbreviations: PK (pseudokinase), nt (nucleotide), UCH (ubiquitin C-terminal hydrolase)
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 (PDF 1760 kb)
Rights and permissions
About this article
Cite this article
Schäfer, I., Rode, M., Bonneau, F. et al. The structure of the Pan2–Pan3 core complex reveals cross-talk between deadenylase and pseudokinase. Nat Struct Mol Biol 21, 591–598 (2014). https://doi.org/10.1038/nsmb.2834
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.2834
This article is cited by
-
Roles of mRNA poly(A) tails in regulation of eukaryotic gene expression
Nature Reviews Molecular Cell Biology (2022)
-
Prospects for pharmacological targeting of pseudokinases
Nature Reviews Drug Discovery (2019)
-
The intrinsic structure of poly(A) RNA determines the specificity of Pan2 and Caf1 deadenylases
Nature Structural & Molecular Biology (2019)
-
PAN3–PSMA2 fusion resulting from a novel t(7;13)(p14;q12) chromosome translocation in a myelodysplastic syndrome that evolved into acute myeloid leukemia
Experimental Hematology & Oncology (2018)
-
USP52 acts as a deubiquitinase and promotes histone chaperone ASF1A stabilization
Nature Communications (2018)