Abstract
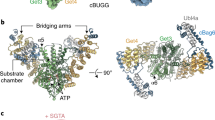
Correct localization of membrane proteins is essential to all cells. Chaperone cascades coordinate the capture and handover of substrate proteins from the ribosomes to the target membranes, yet the mechanistic and structural details of these processes remain unclear. Here we investigate the conserved GET pathway, in which the Get4–Get5 complex mediates the handover of tail-anchor (TA) substrates from the cochaperone Sgt2 to the Get3 ATPase, the central targeting factor. We present a crystal structure of a yeast Get3–Get4–Get5 complex in an ATP-bound state and show how Get4 primes Get3 by promoting the optimal configuration for substrate capture. Structure-guided biochemical analyses demonstrate that Get4-mediated regulation of ATP hydrolysis by Get3 is essential to efficient TA-protein targeting. Analogous regulation of other chaperones or targeting factors could provide a general mechanism for ensuring effective substrate capture during protein biogenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Shao, S. & Hegde, R.S. Membrane protein insertion at the endoplasmic reticulum. Annu. Rev. Cell Dev. Biol. 27, 25–56 (2011).
Kutay, U., Hartmann, E. & Rapoport, T.A. A class of membrane proteins with a C-terminal anchor. Trends Cell Biol. 3, 72–75 (1993).
Kutay, U., Ahnert-Hilger, G., Hartmann, E., Wiedenmann, B. & Rapoport, T.A. Transport route for synaptobrevin via a novel pathway of insertion into the endoplasmic reticulum membrane. EMBO J. 14, 217–223 (1995).
Borgese, N., Gazzoni, I., Barberi, M., Colombo, S. & Pedrazzini, E. Targeting of a tail-anchored protein to endoplasmic reticulum and mitochondrial outer membrane by independent but competing pathways. Mol. Biol. Cell 12, 2482–2496 (2001).
Borgese, N., Brambillasca, S. & Colombo, S. How tails guide tail-anchored proteins to their destinations. Curr. Opin. Cell Biol. 19, 368–375 (2007).
Stefanovic, S. & Hegde, R.S. Identification of a targeting factor for posttranslational membrane protein insertion into the ER. Cell 128, 1147–1159 (2007).
Favaloro, V., Spasic, M., Schwappach, B. & Dobberstein, B. Distinct targeting pathways for the membrane insertion of tail-anchored (TA) proteins. J. Cell Sci. 121, 1832–1840 (2008).
Schuldiner, M. et al. Exploration of the function and organization of the yeast early secretory pathway through an epistatic miniarray profile. Cell 123, 507–519 (2005).
Schuldiner, M. et al. The GET complex mediates insertion of tail-anchored proteins into the ER membrane. Cell 134, 634–645 (2008).
Jonikas, M.C. et al. Comprehensive characterization of genes required for protein folding in the endoplasmic reticulum. Science 323, 1693–1697 (2009).
Battle, A., Jonikas, M.C., Walter, P., Weissman, J.S. & Koller, D. Automated identification of pathways from quantitative genetic interaction data. Mol. Syst. Biol. 6, 379 (2010).
Chartron, J.W., Clemons, W.M. Jr. & Suloway, C.J. The complex process of GETting tail-anchored membrane proteins to the ER. Curr. Opin. Struct. Biol. 22, 217–224 (2012).
Suloway, C.J., Chartron, J.W., Zaslaver, M. & Clemons, W.M. Jr. Model for eukaryotic tail-anchored protein binding based on the structure of Get3. Proc. Natl. Acad. Sci. USA 106, 14849–14854 (2009).
Mateja, A. et al. The structural basis of tail-anchored membrane protein recognition by Get3. Nature 461, 361–366 (2009).
Bozkurt, G. et al. Structural insights into tail-anchored protein binding and membrane insertion by Get3. Proc. Natl. Acad. Sci. USA 106, 21131–21136 (2009).
Hu, J., Li, J., Qian, X., Denic, V. & Sha, B. The crystal structures of yeast Get3 suggest a mechanism for tail-anchored protein membrane insertion. PLoS ONE 4, e8061 (2009).
Yamagata, A. et al. Structural insight into the membrane insertion of tail-anchored proteins by Get3. Genes Cells 15, 29–41 (2010).
Suloway, C.J., Rome, M.E. & Clemons, W.M. Jr. Tail-anchor targeting by a Get3 tetramer: the structure of an archaeal homologue. EMBO J. 31, 707–719 (2012).
Mukhopadhyay, R., Ho, Y.S., Swiatek, P.J., Rosen, B.P. & Bhattacharjee, H. Targeted disruption of the mouse Asna1 gene results in embryonic lethality. FEBS Lett. 580, 3889–3894 (2006).
Mariappan, M. et al. The mechanism of membrane-associated steps in tail-anchored protein insertion. Nature 477, 61–66 (2011).
Stefer, S. et al. Structural basis for tail-anchored membrane protein biogenesis by the Get3-receptor complex. Science 333, 758–762 (2011).
Wang, F., Whynot, A., Tung, M. & Denic, V. The mechanism of tail-anchored protein insertion into the ER membrane. Mol. Cell 43, 738–750 (2011).
Yamamoto, Y. & Sakisaka, T. Molecular machinery for insertion of tail-anchored membrane proteins into the endoplasmic reticulum membrane in mammalian cells. Mol. Cell 48, 387–397 (2012).
Wang, F., Brown, E.C., Mak, G., Zhuang, J. & Denic, V. A chaperone cascade sorts proteins for posttranslational membrane insertion into the endoplasmic reticulum. Mol. Cell 40, 159–171 (2010).
Chartron, J.W., Gonzalez, G.M. & Clemons, W.M. Jr. A structural model of the Sgt2 protein and its interactions with chaperones and the Get4/Get5 complex. J. Biol. Chem. 286, 34325–34334 (2011).
Costanzo, M. et al. The genetic landscape of a cell. Science 327, 425–431 (2010).
Chartron, J.W., Suloway, C.J., Zaslaver, M. & Clemons, W.M. Jr. Structural characterization of the Get4/Get5 complex and its interaction with Get3. Proc. Natl. Acad. Sci. USA 107, 12127–12132 (2010).
Chang, Y.W. et al. Crystal structure of Get4-Get5 complex and its interactions with Sgt2, Get3, and Ydj1. J. Biol. Chem. 285, 9962–9970 (2010).
Chartron, J.W., Vandervelde, D.G., Rao, M. & Clemons, W.M. Jr. The Get5 carboxyl terminal domain is a novel dimerization motif that tethers an extended Get4/Get5 complex. J. Biol. Chem. 287, 8310–8317 (2012).
Chang, Y.W. et al. Interaction surface and topology of Get3-Get4-Get5 protein complex, involved in targeting tail-anchored proteins to endoplasmic reticulum. J. Biol. Chem. 287, 4783–4789 (2012).
Rome, M.E., Rao, M., Clemons, W.M. Jr. & Shan, S.O. Precise timing of ATPase activation drives targeting of tail-anchored proteins. Proc. Natl. Acad. Sci. USA 110, 7666–7671 (2013).
Zhang, X., Rashid, R., Wang, K. & Shan, S.O. Sequential checkpoints govern substrate selection during cotranslational protein targeting. Science 328, 757–760 (2010).
Ataide, S.F. et al. The crystal structure of the signal recognition particle in complex with its receptor. Science 331, 881–886 (2011).
Larkin, M.A. et al. Clustal W and Clustal X version 2.0. Bioinformatics 23, 2947–2948 (2007).
Battye, T.G., Kontogiannis, L., Johnson, O., Powell, H.R. & Leslie, A.G. iMOSFLM: a new graphical interface for diffraction-image processing with MOSFLM. Acta Crystallogr. D Biol. Crystallogr. 67, 271–281 (2011).
Kabsch, W. XDS. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 (2010).
Collaborative Computational Project, Number 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D Biol. Crystallogr. 50, 760–763 (1994).
Winn, M.D. et al. Overview of the CCP4 suite and current developments. Acta Crystallogr. D Biol. Crystallogr. 67, 235–242 (2011).
McCoy, A.J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Adams, P.D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Brunger, A.T. Version 1.2 of the Crystallography and NMR system. Nat. Protoc. 2, 2728–2733 (2007).
Emsley, P., Lohkamp, B., Scott, W.G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Sikorski, R.S. & Hieter, P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122, 19–27 (1989).
Gietz, R.D. & Schiestl, R.H. High-efficiency yeast transformation using the LiAc/SS carrier DNA/PEG method. Nat. Protoc. 2, 31–34 (2007).
Pettersen, E.F. et al. UCSF Chimera: a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Baker, N.A., Sept, D., Joseph, S., Holst, M.J. & McCammon, J.A. Electrostatics of nanosystems: application to microtubules and the ribosome. Proc. Natl. Acad. Sci. USA 98, 10037–10041 (2001).
Dolinsky, T.J., Nielsen, J.E., McCammon, J.A. & Baker, N.A. PDB2PQR: an automated pipeline for the setup of Poisson-Boltzmann electrostatics calculations. Nucleic Acids Res. 32, W665–W667 (2004).
Acknowledgements
We are grateful to V. Denic (Harvard University) for providing the Δget3 Δget5 yeast strain. We thank G. Card, A. Gonzalez and M. Soltis for help with data collection at the Stanford Synchrotron Radiation Lightsource (SSRL) beamline 12-2. We are grateful to G. Moore and B. Moore for support of the Molecular Observatory at the California Institute of Technology. Operations at the SSRL are supported by the US Department of Energy and US National Institutes of Health (NIH). This work was supported by career awards from the David and Lucile Packard Foundation and the Henry Dreyfus Foundation (S.S.), US National Science Foundation Graduate Research Fellowship DGE-1144469 (M.E.R.), NIH training grant 5T32GM007616-33 (H.B.G. and M.R.) and NIH research grant R01GM097572 (W.M.C. Jr.).
Author information
Authors and Affiliations
Contributions
H.B.G. performed all experiments except those noted. M.R. performed Get4 inhibition studies, and M.E.R. performed translocation experiments, both supervised by S.S. J.W.C. initiated the project and aided in refinement. S.S. contributed to experimental design and interpretation. W.M.C. Jr. conceived and supervised the project. H.B.G. and W.M.C. Jr. wrote the manuscript. All authors discussed the results and implications and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Representative ITC isotherms for nucleotide-dependent complex formation.
(a) Representative measurements for Get3 in various nucleotide states. Raw data are shown in the top panel of each trial represented as the power input into the sample cell over time. Integrated data are shown in the bottom panels in terms of the total energy required for equilibration as a function of the molar ratio of Get4-5N:Get3. The solid line represents the best-fit model used to calculate affinities. The affinities indicated for each nucleotide state are the average from at least three experiments. In all experiments Get4-5N was titrated into the sample cell containing Get3. (b) Representative measurements for Get3 mutants from the Anchoring interface, top, or the Regulatory interface, bottom. The indicated affinity for each mutant represents the value obtained from a single experiment carried out in the presence of ATP. (c) Representative measurements for Get4 mutants from the various interfaces as in (b).
Supplementary Figure 2 Surface properties of Get3 and Get4.
(a) Left, Surface representation of closed Get3 dimer (gray) from (b) showing Get4 binding site (blue), Get1 cytoplasmic domain binding site (yellow) and the overlap (green). Right, Similar surface representation highlighting the Get4 binding site (blue), Get2 cytoplasmic domain binding site (red) and the overlap (brown). (b) Left, orientation of Get3 used in subsequent panels to highlight the binding interfaces. Monomers are colored as in Figure 2. For surface representations one monomer is outlined in black and dashed lines (blue) highlight the interaction surface of Get4 on Get3. Center, accessible surface color ramped based on electrostatic potential from -12 kT/e (red) to +12 kT/e (blue). Get5N colored gray. Right, accessible surface color ramped based on conservation from ≤50% (white) to ≥90% (purple). Get5N colored gray. Coloring is based on a ClustalW 34 alignment using the following sequences: Saccharomyces cerevisiae, Schizosaccharomyces pombe, Aspergillus fumigatus, Candida albicans, Pichia pastoris, Nematostella vectensis, Caenorhabditis elegans, Drosophila melanogaster, Danio rerio, Xenopus laevis, Mus musculus, Homo sapiens, Arabidopsis thaliana, Neurospora crassa, Anolis carolinensis. (c) Surface properties of Get4 as in (b), except Get4 is color-ramped from N-terminus (blue) to C-terminus (red).
Supplementary Figure 3 Stereo views of the Get3D-Get4–5N interface.
(a) The Get3D-Get4 interface. Coloring is the same as in Figure 3a. Get3 Lys69 and Get4 Asp74 are highlighted by open arrowheads. (b) 2FO-FC Electron density contoured at 1.5σ highlighting the Get3D-Get4 interface.
Supplementary Figure 4 TA targeting assay.
(a) Representative autoradiograph images used to calculate Get3 translocation efficiency corresponding to Figure 4b for wildtype (Top) and K69D (Bottom). (b) Comparison of Get3 translocation efficiencies in Δget3 and Δget3/Δmdy2 lysates. (c) Representative autoradiograph images used to calculate Get3 translocation efficiencies for Δget3/Δmdy2 lysate in (b).
Supplementary Figure 5 Tetramers of Get3.
(a) Tetramers of Get3 seen in various crystal forms with Get3 dimers similar to Figure 5a,b. Each is highlighted with the archaeal Get3 (Methanocaldococcus jannaschii) from PDB ID 3UG6, the Get2-Get3 complex from PDB ID 3SJD, and the Get3D-Get4-5N crystal structure. (b) Stereo view of the 2Fo-Fc density contoured at 1.5σ showing the loops involved in formation of the Get3D-Get4-5N complex tetramer. (c) Comparison of the Archaeal MjGet3 dimer aligned to one dimer of the Get3D-Get4-5N complex dimer.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 and Supplementary Table 1 (PDF 64948 kb)
Rights and permissions
About this article
Cite this article
Gristick, H., Rao, M., Chartron, J. et al. Crystal structure of ATP-bound Get3–Get4–Get5 complex reveals regulation of Get3 by Get4. Nat Struct Mol Biol 21, 437–442 (2014). https://doi.org/10.1038/nsmb.2813
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.2813
This article is cited by
-
Dynamic stability of Sgt2 enables selective and privileged client handover in a chaperone triad
Nature Communications (2024)
-
The Ways of Tails: the GET Pathway and more
The Protein Journal (2019)
-
Transcriptional regulation of secretory capacity by bZip transcription factors
Frontiers in Biology (2015)