Abstract
Tyrosine kinase 2 (TYK2) is a member of the Janus kinase (JAK) family of nonreceptor tyrosine kinases, which are essential for proper signaling in immune responses and development. Here we present a 2.0-Å-resolution crystal structure of a receptor-binding fragment of human TYK2, encompassing the FERM and SH2 domains, in complex with a so-called 'box2'-containing intracellular peptide motif from the interferon-α receptor chain 1 (IFNAR1). The TYK2-IFNAR1 interface reveals an unexpected receptor-binding mode that mimics a SH2 domain–phosphopeptide interaction, with a glutamate replacing the canonical phosphotyrosine residue. This structure provides the first view, to our knowledge, of a JAK in complex with its cognate receptor and defines the molecular logic through which JAKs have evolved to interact with divergent receptor sequences.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Leonard, W.J. & O'Shea, J.J. Jaks and STATs: biological implications. Annu. Rev. Immunol. 16, 293–322 (1998).
Haan, C., Kreis, S., Margue, C. & Behrmann, I. Jaks and cytokine receptors: an intimate relationship. Biochem. Pharmacol. 72, 1538–1546 (2006).
Casanova, J.L., Holland, S.M. & Notarangelo, L.D. Inborn errors of human JAKs and STATs. Immunity 36, 515–528 (2012).
Kontzias, A., Kotlyar, A., Laurence, A., Changelian, P. & O'Shea, J.J. Jakinibs: a new class of kinase inhibitors in cancer and autoimmune disease. Curr. Opin. Pharmacol. 12, 464–470 (2012).
Radtke, S. et al. The Jak1 SH2 domain does not fulfill a classical SH2 function in Jak/STAT signaling but plays a structural role for receptor interaction and up-regulation of receptor surface expression. J. Biol. Chem. 280, 25760–25768 (2005).
Richter, M.F., Dumenil, G., Uze, G., Fellous, M. & Pellegrini, S. Specific contribution of Tyk2 JH regions to the binding and the expression of the interferon α/β receptor component IFNAR1. J. Biol. Chem. 273, 24723–24729 (1998).
Zhao, Y., Wagner, F., Frank, S.J. & Kraft, A.S. The amino-terminal portion of the JAK2 protein kinase is necessary for binding and phosphorylation of the granulocyte-macrophage colony-stimulating factor receptor β chain. J. Biol. Chem. 270, 13814–13818 (1995).
Chen, M. et al. The amino terminus of JAK3 is necessary and sufficient for binding to the common γ chain and confers the ability to transmit interleukin 2-mediated signals. Proc. Natl. Acad. Sci. USA 94, 6910–6915 (1997).
Cacalano, N.A. et al. Autosomal SCID caused by a point mutation in the N-terminus of Jak3: mapping of the Jak3-receptor interaction domain. EMBO J. 18, 1549–1558 (1999).
Murakami, M. et al. Critical cytoplasmic region of the interleukin-6 signal transducer gp130 is conserved in the cytokine receptor family. Proc. Natl. Acad. Sci. USA 88, 11349–11353 (1991).
Pelletier, S., Gingras, S., Funakoshi-Tago, M., Howell, S. & Ihle, J.N. Two domains of the erythropoietin receptor are sufficient for Jak2 binding/activation and function. Mol. Cell. Biol. 26, 8527–8538 (2006).
Lebrun, J.J., Ali, S., Ullrich, A. & Kelly, P.A. Proline-rich sequence-mediated Jak2 association to the prolactin receptor is required but not sufficient for signal transduction. J. Biol. Chem. 270, 10664–10670 (1995).
Yan, H., Krishnan, K., Lim, J.T., Contillo, L.G. & Krolewski, J.J. Molecular characterization of an alpha interferon receptor 1 subunit (IFNaR1) domain required for TYK2 binding and signal transduction. Mol. Cell. Biol. 16, 2074–2082 (1996).
Tanner, J.W., Chen, W., Young, R.L., Longmore, G.D. & Shaw, A.S. The conserved box 1 motif of cytokine receptors is required for association with JAK kinases. J. Biol. Chem. 270, 6523–6530 (1995).
Usacheva, A. et al. Contribution of the Box 1 and Box 2 motifs of cytokine receptors to Jak1 association and activation. J. Biol. Chem. 277, 48220–48226 (2002).
Royer, Y., Staerk, J., Costuleanu, M., Courtoy, P.J. & Constantinescu, S.N. Janus kinases affect thrombopoietin receptor cell surface localization and stability. J. Biol. Chem. 280, 27251–27261 (2005).
Haan, C., Heinrich, P.C. & Behrmann, I. Structural requirements of the interleukin-6 signal transducer gp130 for its interaction with Janus kinase 1: the receptor is crucial for kinase activation. Biochem. J. 361, 105–111 (2002).
Haan, C., Hermanns, H.M., Heinrich, P.C. & Behrmann, I. A single amino acid substitution (Trp666→Ala) in the interbox1/2 region of the interleukin-6 signal transducer gp130 abrogates binding of JAK1, and dominantly impairs signal transduction. Biochem. J. 349, 261–266 (2000).
Bogdan, C. The function of type I interferons in antimicrobial immunity. Curr. Opin. Immunol. 12, 419–424 (2000).
García-Sastre, A. & Biron, C.A. Type 1 interferons and the virus-host relationship: a lesson in detente. Science 312, 879–882 (2006).
Dunn, G.P., Koebel, C.M. & Schreiber, R.D. Interferons, immunity and cancer immunoediting. Nat. Rev. Immunol. 6, 836–848 (2006).
Theofilopoulos, A.N., Baccala, R., Beutler, B. & Kono, D.H. Type I interferons (α/β) in immunity and autoimmunity. Annu. Rev. Immunol. 23, 307–336 (2005).
Velazquez, L., Fellous, M., Stark, G.R. & Pellegrini, S. A protein tyrosine kinase in the interferon α/β signaling pathway. Cell 70, 313–322 (1992).
Colamonici, O. et al. Direct binding to and tyrosine phosphorylation of the alpha subunit of the type I interferon receptor by p135tyk2 tyrosine kinase. Mol. Cell. Biol. 14, 8133–8142 (1994).
Colamonici, O.R., Uyttendaele, H., Domanski, P., Yan, H. & Krolewski, J.J. p135tyk2, an interferon-α-activated tyrosine kinase, is physically associated with an interferon-α receptor. J. Biol. Chem. 269, 3518–3522 (1994).
Ragimbeau, J. et al. The tyrosine kinase Tyk2 controls IFNAR1 cell surface expression. EMBO J. 22, 537–547 (2003).
Lupardus, P.J. et al. Structural snapshots of full-length Jak1, a transmembrane gp130/IL-6/IL-6Rα cytokine receptor complex, and the receptor-Jak1 holocomplex. Structure 19, 45–55 (2011).
Smith, W.J., Nassar, N., Bretscher, A., Cerione, R.A. & Karplus, P.A. Structure of the active N-terminal domain of Ezrin: conformational and mobility changes identify keystone interactions. J. Biol. Chem. 278, 4949–4956 (2003).
Hamada, K., Shimizu, T., Matsui, T., Tsukita, S. & Hakoshima, T. Structural basis of the membrane-targeting and unmasking mechanisms of the radixin FERM domain. EMBO J. 19, 4449–4462 (2000).
Pearson, M.A., Reczek, D., Bretscher, A. & Karplus, P.A. Structure of the ERM protein moesin reveals the FERM domain fold masked by an extended actin binding tail domain. Cell 101, 259–270 (2000).
Ceccarelli, D.F., Song, H.K., Poy, F., Schaller, M.D. & Eck, M.J. Crystal structure of the FERM domain of focal adhesion kinase. J. Biol. Chem. 281, 252–259 (2006).
Eck, M.J., Shoelson, S.E. & Harrison, S.C. Recognition of a high-affinity phosphotyrosyl peptide by the Src homology-2 domain of p56lck. Nature 362, 87–91 (1993).
Yang, J. et al. Crystal structure of human protein-tyrosine phosphatase SHP-1. J. Biol. Chem. 278, 6516–6520 (2003).
Lee, C.H. et al. Crystal structures of peptide complexes of the amino-terminal SH2 domain of the Syp tyrosine phosphatase. Structure 2, 423–438 (1994).
Bradshaw, J.M. & Waksman, G. Molecular recognition by SH2 domains. Adv. Protein Chem. 61, 161–210 (2002).
Poy, F. et al. Crystal structures of the XLP protein SAP reveal a class of SH2 domains with extended, phosphotyrosine-independent sequence recognition. Mol. Cell 4, 555–561 (1999).
Yoh, S.M., Cho, H., Pickle, L., Evans, R.M. & Jones, K.A. The Spt6 SH2 domain binds Ser2-P RNAPII to direct Iws1-dependent mRNA splicing and export. Genes Dev. 21, 160–174 (2007).
Kaneko, T. et al. Loops govern SH2 domain specificity by controlling access to binding pockets. Sci. Signal. 3, ra34 (2010).
Moser, M., Legate, K.R., Zent, R. & Fassler, R. The tail of integrins, talin, and kindlins. Science 324, 895–899 (2009).
Pearlman, S.M., Serber, Z. & Ferrell, J.E. Jr. A mechanism for the evolution of phosphorylation sites. Cell 147, 934–946 (2011).
Liu, B.A. et al. The human and mouse complement of SH2 domain proteins-establishing the boundaries of phosphotyrosine signaling. Mol. Cell 22, 851–868 (2006).
Pantoliano, M.W. et al. High-density miniaturized thermal shift assays as a general strategy for drug discovery. J. Biomol. Screen. 6, 429–440 (2001).
Niesen, F.H., Berglund, H. & Vedadi, M. The use of differential scanning fluorimetry to detect ligand interactions that promote protein stability. Nat. Protoc. 2, 2212–2221 (2007).
Kabsch, W. Xds. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 (2010).
Potterton, E., Briggs, P., Turkenburg, M. & Dodson, E. A graphical user interface to the CCP4 program suite. Acta Crystallogr. D Biol. Crystallogr. 59, 1131–1137 (2003).
Adams, P.D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Chen, V.B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 66, 12–21 (2010).
Krissinel, E. & Henrick, K. Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 372, 774–797 (2007).
Baker, N.A., Sept, D., Joseph, S., Holst, M.J. & McCammon, J.A. Electrostatics of nanosystems: application to microtubules and the ribosome. Proc. Natl. Acad. Sci. USA 98, 10037–10041 (2001).
Ashkenazy, H., Erez, E., Martz, E., Pupko, T. & Ben-Tal, N. ConSurf 2010: calculating evolutionary conservation in sequence and structure of proteins and nucleic acids. Nucleic Acids Res. 38, W529–W533 (2010).
Do, C.B., Mahabhashyam, M.S., Brudno, M. & Batzoglou, S. ProbCons: probabilistic consistency-based multiple sequence alignment. Genome Res. 15, 330–340 (2005).
Acknowledgements
We thank X. Ma, M. Ultsch, J. Payandeh, L. Wu and the staff at the Stanford Synchrotron Radiation Lightsource (SSRL) for their technical advice and assistance. We also thank the Structural Biology Expression Group, Microchemistry and Proteomics Laboratory and DNA Sequencing facilities at Genentech for their technical support. Portions of this research were carried out at the SSRL, a Directorate of SLAC National Accelerator Laboratory and an Office of Science User Facility operated for the US Department of Energy (DOE) Office of Science by Stanford University. The SSRL Structural Molecular Biology Program is supported by the DOE Office of Biological and Environmental Research and by the US National Institutes of Health (NIH), National Institute of General Medical Sciences (NIGMS; including P41GM103393). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of NIGMS or NIH.
Author information
Authors and Affiliations
Contributions
H.J.A.W. performed protein purification, crystallization and biochemical experiments. C.T. and Y.F. performed design and cloning of TYK2 constructs. H.J.A.W. and M.A.S. participated in data analysis and manuscript preparation. P.J.L. solved and analyzed the structure, prepared figures and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Biochemical analysis of the TYK2–IFNAR1 complex
Comparison of size exclusion chromatograms of (a) the TYK2 FERM–SH2 to the (b) TYK2 FERM–SH2+IFNAR1 single chain fusion. Recombinant TYK2 FERM–SH2 (residues 23–566) or TYK2 FERM–SH2 (residues 23–583) fused to IFNAR1 (residues 465–512) were expressed in insect cells, purified by Ni-affinity and analyzed by size exclusion chromatography on a Superdex 200 size exclusion column. Labeled fractions were then analyzed by SDS-PAGE. The solution behavior and monodispersity of TYK2 FERM–SH2 without IFNAR1 was compromised when compared to the correctly sized monodisperse peak seen when IFNAR1 chain was present. (c) Size exclusion chromatogram showing co-elution of TYK2 FERM–SH2 (residues 23–583) and IFNAR1 peptide (residues 465–512) after protease-mediated cleavage of the linker sequence. Marked fractions were analyzed by SDS-PAGE, and “L” denotes load sample. Protein standards are overlaid on the chromatogram and shown as a gray dashed line. (d) LC/MS analysis of TYK2 non-covalently associated with IFNAR1. A peak fraction from the purification of the TEV protease-cleaved TYK2/IFNAR1 complex shown in (c) was analyzed by HPLC followed by MS-TOF to determine intact mass of the protein contents of that fraction. Two peaks were observed, the first corresponding to IFNAR1 peptide (top panel) and the second corresponding to the TYK2 FERM/SH2 (bottom panel). The sequence of the TEV-cleavable TYK2/IFNAR1 construct analyzed is shown underneath the chromatogram in (c).
Supplementary Figure 2 Structural analysis and crystal packing of the TYK2–IFNAR1 complex
(a) Stereo view cartoon diagram of the TYK2 FERM–SH2 in complex with IFNAR1. TYK2 is colored as a rainbow, with blue starting at the N-terminus to red at the C-terminus. IFNAR1 is colored yellow. (b) Stereo view of electron density (2Fobs-Fcalc contoured at 1σ) around the SH2 domain and IFNAR1 peptide. The backbone is shown as a ribbon with sidechains shown as stick models, with TYK2 colored as in (a). (c) Analysis of crystal packing and contacts near the IFNAR1 peptide. Front view and side view of the TYK2–IFNAR1 complex showing nearest neighbors to IFNAR1 in the crystal lattice. IFNAR1 does not participate in any crystal contacts, indicating the peptide is bound in the native conformation.
Supplementary Figure 3 Secondary structure of the TYK2 FERM-SH2 domain, and comparison of TYK2 primary sequence to the other human JAK family members
Secondary structure elements are labeled using conventions established for the FAK FERM domain and SH2 domain of Lck. Secondary structural elements are displayed above the alignments, with helices displayed as cylinders, strands displayed as block arrows, and loops displayed as lines. Secondary structure is labeled and domain structure colors matched between the structure and the alignment. Residues contacting IFNAR1 are shaded in yellow.
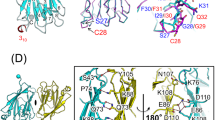
Supplementary Figure 4 Details of the TYK2 FERM-SH2 interdomain linker structure and SH2 domain comparison
(a) Close up view showing the L1 linker connecting the F1 and F2 subdomains of the FERM domain along with the C-terminal L3 linker. (b) Close-up view showing the L2 linker that connects the FERM F3 subdomain to the SH2 domain. All linkers are shown in orange, with key residues shown as stick models. (c-e) Comparison of the TYK2 SH2 domain to the SHP2 N-SH2. Annotated view of the (c) TYK2 SH2 (blue) and (d) SHP2 N-SH2 (PDB ID 1AYB, purple) secondary structure elements, to illustrate similarities and differences between the TYK2 SH2 and a canonical phosphopeptide-binding SH2 domain. (e) Overlay of the SHP2 N-SH2 domain onto the TYK2 SH2 domain (RMSD 2.0Å over 89 residues). Alignments generated using SUPERPOSE.
Supplementary Figure 5 Differential scanning fluorimetry analysis of single-chain TYK2–IFNAR1 wild-type and mutant complexes
Curves are plotted and overlaid for each individual experiment, with apparent melting temperature (Tm), standard deviation (SD), P-value (calculated by unpaired, two-tailed students t-test) comparing the mutants against wild type, and number of samples (n) are printed below each graph. At the bottom, representative fitted curves are plotted and overlaid for each of the proteins to illustrate the experimental curve differences between the wild type and IFNAR1 mutants.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 and Supplementary Table 1 (PDF 1062 kb)
Source data
Rights and permissions
About this article
Cite this article
Wallweber, H., Tam, C., Franke, Y. et al. Structural basis of recognition of interferon-α receptor by tyrosine kinase 2. Nat Struct Mol Biol 21, 443–448 (2014). https://doi.org/10.1038/nsmb.2807
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.2807
This article is cited by
-
Double-edged sword of JAK/STAT signaling pathway in viral infections: novel insights into virotherapy
Cell Communication and Signaling (2023)
-
STAM and Hrs interact sequentially with IFN-α Receptor to control spatiotemporal JAK–STAT endosomal activation
Nature Cell Biology (2023)
-
A typical acute lymphoblastic leukemia JAK2 variant, R683G, causes an aggressive form of familial thrombocytosis when germline
Leukemia (2021)
-
Orchestration of signaling by structural disorder in class 1 cytokine receptors
Cell Communication and Signaling (2020)
-
Are peptides a solution for the treatment of hyperactivated JAK3 pathways?
Inflammopharmacology (2019)