Abstract
Chromatin ubiquitylation flanking DNA double-strand breaks (DSBs), mediated by RNF8 and RNF168 ubiquitin ligases, orchestrates a two-branch pathway, recruiting repair factors 53BP1 or the RAP80–BRCA1 complex. We report that human demethylase JMJD1C regulates the RAP80–BRCA1 branch of this DNA-damage response (DDR) pathway. JMJD1C was stabilized by interaction with RNF8, was recruited to DSBs, and was required for local ubiquitylations and recruitment of RAP80–BRCA1 but not 53BP1. JMJD1C bound to RNF8 and MDC1, and demethylated MDC1 at Lys45, thereby promoting MDC1-RNF8 interaction, RNF8-dependent MDC1 ubiquitylation and recruitment of RAP80–BRCA1 to polyubiquitylated MDC1. Furthermore, JMJD1C restricted formation of RAD51 repair foci, and JMJD1C depletion caused resistance to ionizing radiation and PARP inhibitors, phenotypes relevant to aberrant loss of JMJD1C in subsets of breast carcinomas. These findings identify JMJD1C as a DDR component, with implications for genome-integrity maintenance, tumorigenesis and cancer treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Ciccia, A. & Elledge, S.J. The DNA damage response: making it safe to play with knives. Mol. Cell 40, 179–204 (2010).
van Attikum, H. & Gasser, S.M. Crosstalk between histone modifications during the DNA damage response. Trends Cell Biol. 19, 207–217 (2009).
Lukas, J., Lukas, C. & Bartek, J. More than just a focus: the chromatin response to DNA damage and its role in genome integrity maintenance. Nat. Cell Biol. 13, 1161–1169 (2011).
Huen, M.S. et al. RNF8 transduces the DNA-damage signal via histone ubiquitylation and checkpoint protein assembly. Cell 131, 901–914 (2007).
Kolas, N.K. et al. Orchestration of the DNA-damage response by the RNF8 ubiquitin ligase. Science 318, 1637–1640 (2007).
Mailand, N. et al. RNF8 ubiquitylates histones at DNA double-strand breaks and promotes assembly of repair proteins. Cell 131, 887–900 (2007).
Doil, C. et al. RNF168 binds and amplifies ubiquitin conjugates on damaged chromosomes to allow accumulation of repair proteins. Cell 136, 435–446 (2009).
Stewart, G.S. et al. The RIDDLE syndrome protein mediates a ubiquitin-dependent signaling cascade at sites of DNA damage. Cell 136, 420–434 (2009).
Panier, S. et al. Tandem protein interaction modules organize the ubiquitin-dependent response to DNA double-strand breaks. Mol. Cell 47, 383–395 (2012).
Mattiroli, F. et al. RNF168 ubiquitinates K13–15 on H2A/H2AX to drive DNA damage signaling. Cell 150, 1182–1195 (2012).
Bothmer, A. et al. 53BP1 regulates DNA resection and the choice between classical and alternative end joining during class switch recombination. J. Exp. Med. 207, 855–865 (2010).
Bouwman, P. et al. 53BP1 loss rescues BRCA1 deficiency and is associated with triple-negative and BRCA-mutated breast cancers. Nat. Struct. Mol. Biol. 17, 688–695 (2010).
Bunting, S.F. et al. 53BP1 inhibits homologous recombination in Brca1-deficient cells by blocking resection of DNA breaks. Cell 141, 243–254 (2010).
Coleman, K.A. & Greenberg, R.A. The BRCA1–RAP80 complex regulates DNA repair mechanism utilization by restricting end resection. J. Biol. Chem. 286, 13669–13680 (2011).
Hu, Y. et al. RAP80-directed tuning of BRCA1 homologous recombination function at ionizing radiation-induced nuclear foci. Genes Dev. 25, 685–700 (2011).
Fradet-Turcotte, A. et al. 53BP1 is a reader of the DNA-damage-induced H2A Lys 15 ubiquitin mark. Nature 499, 50–54 (2013).
Acs, K. et al. The AAA-ATPase VCP/p97 promotes 53BP1 recruitment by removing L3MBTL1 from DNA double-strand breaks. Nat. Struct. Mol. Biol. 18, 1345–1350 (2011).
Meerang, M. et al. The ubiquitin-selective segregase VCP/p97 orchestrates the response to DNA double-strand breaks. Nat. Cell Biol. 13, 1376–1382 (2011).
Strauss, C. & Goldberg, M. Recruitment of proteins to DNA double-strand breaks: MDC1 directly recruits RAP80. Cell Cycle 10, 2850–2857 (2011).
Mallette, F.A. et al. RNF8- and RNF168-dependent degradation of KDM4A/JMJD2A triggers 53BP1 recruitment to DNA damage sites. EMBO J. 31, 1865–1878 (2012).
Difilippantonio, S. et al. 53BP1 facilitates long-range DNA end-joining during V(D)J recombination. Nature 456, 529–533 (2008).
Dimitrova, N., Chen, Y.C., Spector, D.L. & de Lange, T. 53BP1 promotes non-homologous end joining of telomeres by increasing chromatin mobility. Nature 456, 524–528 (2008).
Danielsen, J.M. et al. Mass spectrometric analysis of lysine ubiquitylation reveals promiscuity at site level. Mol Cell Proteomics 10, M110 003590 (2011).
Yang, W.-L. et al. The E3 ligase TRAF6 regulates Akt ubiquitination and activation. Science 325, 1134–1138 (2009).
Kim, S.M. et al. Regulation of mouse steroidogenesis by WHISTLE and JMJD1C through histone methylation balance. Nucleic Acids Res. 38, 6389–6403 (2010).
Kim, H., Chen, J. & Yu, X. Ubiquitin-binding protein RAP80 mediates BRCA1-dependent DNA damage response. Science 316, 1202–1205 (2007).
Sobhian, B. et al. RAP80 targets BRCA1 to specific ubiquitin structures at DNA damage sites. Science 316, 1198–1202 (2007).
Wang, B. et al. Abraxas and RAP80 form a BRCA1 protein complex required for the DNA damage response. Science 316, 1194–1198 (2007).
Yan, J. et al. The ubiquitin-interacting motif containing protein RAP80 interacts with BRCA1 and functions in DNA damage repair response. Cancer Res. 67, 6647–6656 (2007).
Wolf, S.S., Patchev, V.K. & Obendorf, M. A novel variant of the putative demethylase gene, s-JMJD1C, is a coactivator of the AR. Arch. Biochem. Biophys. 460, 56–66 (2007).
Zhang, X., Wen, H. & Shi, X. Lysine methylation: beyond histones. Acta Biochim. Biophys. Sin. (Shanghai) 44, 14–27 (2012).
Choudhary, C. et al. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science 325, 834–840 (2009).
Strauss, C., Halevy, T., Macarov, M., Argaman, L. & Goldberg, M. MDC1 is ubiquitylated on its tandem BRCT domain and directly binds RAP80 in a UBC13-dependent manner. DNA Repair (Amst.) 10, 806–814 (2011).
Galanty, Y., Belotserkovskaya, R., Coates, J. & Jackson, S.P. RNF4, a SUMO-targeted ubiquitin E3 ligase, promotes DNA double-strand break repair. Genes Dev. 26, 1179–1195 (2012).
Luo, K., Zhang, H., Wang, L., Yuan, J. & Lou, Z. Sumoylation of MDC1 is important for proper DNA damage response. EMBO J. 31, 3008–3019 (2012).
Yin, Y. et al. SUMO-targeted ubiquitin E3 ligase RNF4 is required for the response of human cells to DNA damage. Genes Dev. 26, 1196–1208 (2012).
Hu, X., Paul, A. & Wang, B. Rap80 protein recruitment to DNA double-strand breaks requires binding to both small ubiquitin-like modifier (SUMO) and ubiquitin conjugates. J. Biol. Chem. 287, 25510–25519 (2012).
Lou, Z., Minter-Dykhouse, K., Wu, X. & Chen, J. MDC1 is coupled to activated CHK2 in mammalian DNA damage response pathways. Nature 421, 957–961 (2003).
Joosse, S.A. BRCA1 and BRCA2: a common pathway of genome protection but different breast cancer subtypes. Nat. Rev. Cancer 12, 372 (2012).
Adamson, B., Smogorzewska, A., Sigoillot, F.D., King, R.W. & Elledge, S.J. A genome-wide homologous recombination screen identifies the RNA-binding protein RBMX as a component of the DNA-damage response. Nat. Cell Biol. 14, 318–328 (2012).
Bartkova, J. et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature 434, 864–870 (2005).
Bryant, H.E. et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 434, 913–917 (2005).
Farmer, H. et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 434, 917–921 (2005).
Coster, G. & Goldberg, M. The cellular response to DNA damage: a focus on MDC1 and its interacting proteins. Nucleus 1, 166–178 (2010).
Jorgensen, S. et al. SET8 is degraded via PCNA-coupled CRL4(CDT2) ubiquitylation in S phase and after UV irradiation. J. Cell Biol. 192, 43–54 (2011).
Takahashi, A. et al. DNA damage signaling triggers degradation of histone methyltransferases through APC/C(Cdh1) in senescent cells. Mol. Cell 45, 123–131 (2012).
Callen, E. et al. 53BP1 mediates productive and mutagenic DNA repair through distinct phosphoprotein interactions. Cell 153, 1266–1280 (2013).
Chapman, J.R. et al. RIF1 is essential for 53BP1-dependent nonhomologous end joining and suppression of DNA double-strand break resection. Mol. Cell 49, 858–871 (2013).
Di Virgilio, M. et al. Rif1 prevents resection of DNA breaks and promotes immunoglobulin class switching. Science 339, 711–715 (2013).
Escribano-Díaz, C. et al. A cell cycle-dependent regulatory circuit composed of 53BP1–RIF1 and BRCA1-CtIP controls DNA repair pathway choice. Mol. Cell 49, 872–883 (2013).
Zimmermann, M., Lottersberger, F., Buonomo, S.B., Sfeir, A. & de Lange, T. 53BP1 regulates DSB repair using Rif1 to control 5′ end resection. Science 339, 700–704 (2013).
Gudjonsson, T. et al. TRIP12 and UBR5 suppress spreading of chromatin ubiquitylation at damaged chromosomes. Cell 150, 697–709 (2012).
Shevchenko, A., Tomas, H., Havlis, J., Olsen, J.V. & Mann, M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 1, 2856–2860 (2006).
Rappsilber, J., Ishihama, Y. & Mann, M. Stop and go extraction tips for matrix-assisted laser desorption/ionization, nanoelectrospray, and LC/MS sample pretreatment in proteomics. Anal. Chem. 75, 663–670 (2003).
Kelstrup, C.D., Young, C., Lavallee, R., Nielsen, M.L. & Olsen, J.V. Optimized fast and sensitive acquisition methods for shotgun proteomics on a quadrupole Orbitrap mass spectrometer. J. Proteome Res. 11, 3487–3497 (2012).
Cox, J. & Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 26, 1367–1372 (2008).
Ressler, S. et al. p16INK4A is a robust in vivo biomarker of cellular aging in human skin. Aging Cell 5, 379–389 (2006).
Acknowledgements
We thank C. Lukas, R. Strauss and C. Dinant for technical advice, and the Danish Cancer Society (R56-A3237-12-S2, to J. Bartek), the Novo Nordisk Foundation (#2290, to J. Bartek), the Danish Council for Independent Research (DFF-1331-00262, to J. Bartek and B.B.), the Lundbeck Foundation (R93-A89990, to J. Bartek), and the European Community projects Biomedreg (CZ.1.05/2.1.00/01.0030, to J. Bartek) and DDResponse (259893, to J. Bartkova and J. Bartek) for funding.
Author information
Authors and Affiliations
Contributions
S.W., K.W., J.L. and J. Bartek designed the project. S.W. and K.W. performed the majority of biochemical and cell biology experiments. K.W., V.A., B.B. and J.L. provided the SILAC screen data. S.W. and J. Bartek generated the JMJD1C antibody (DCS-410). S.W., V.A. and B.B. performed the mass spectrometry analysis. J. Bartkova and J. Bartek performed immunohistochemical analyses and evaluations. S.W., J.L. and J. Bartek wrote the manuscript. All authors discussed and interpreted the data and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
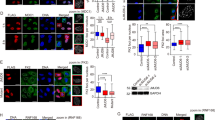
Supplementary Figure 1 JMJD1C is expressed in diverse human cell types and is regulated by RNF8 and RNF168 through the ubiquitin-proteasome pathway.
(a) JMJD1C mRNA expression was analyzed by quantitative real-time RT-PCR in 9 human cell types. The values were normalized to β-actin mRNA. (b) U2OS cells were cotransfected with Flag-tagged fragment B of JMJD1C and siRNA oligonucleotides targeting control or E3 enzymes RNF8, RNF168, BRCA1, DZIP3 and BBAP, respectively; incubated for 72 h, and whole-cell extracts analyzed by immunoblotting using indicated antibodies. More than 90% of knockdown efficiency of DZIP3 and BBAP was confirmed by realtime RT-PCR (data not shown). (c) HEK 293T cells were transfected with siRNA oligonucleotides targeting control, RNF8 and/or RNF168, and next day transfected with Flag-tagged JMJD1C fragment B, mock or Myc-tagged Ubiquitin, and after additional incubation for 2 days cells were used for in vivo ubiquitylation assay. (d) HEK 293T cells were cotransfected with Flag-tagged JMJD1C fragment B and HA-tagged Ubiquitin (mock, WT, K48-only or K63-only) as indicated, and after incubation for 2 days cells were used for in vivo ubiquitylation assay. Asterisk indicates non specific bands.
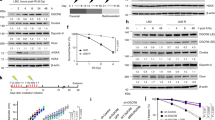
Supplementary Figure 2 JMJD1C expression is specifically depleted by JMJD1C-targeting siRNA oligonucleotides, and catalytic domain and RNF8-binding domain of JMJD1C are essential for accumulation of JMJD1C at DNA-damage sites.
(a) U2OS cells were transfected with control or 2 kinds of JMJD1C-targeting siRNA oligonucleotides, incubated for 72 h, and analyzed by immunoblotting with the indicated antibodies. (b) U2OS cell lines stably expressing Flag-mock or siRNA-insensitive Flag-JMJD1C-WT, -ΔB, -H2336A and -ΔB-H2336A were transfected with control or JMJD1C-targeting siRNA, incubated for 72 h, and analyzed by immunoblotting with the indicated antibodies. (c) U2OS cell lines stably expressing Flag-JMJD1C-WT, -ΔB and -H2336A were co-immunostained with antibodies to Flag and γ-H2AX at 10 minutes after exposure to laser micro irradiation. (d) Accumulation of JMJD1C protein at microlaser-generated lesions in U2OS cells, as detected by immunofluorescence, is attenuated by siRNA-mediated depletion of either RNF8 or RNF168 alone, and it is completely abolished in cells with concomitant knock-down of RNF8 and RNF168.
Supplementary Figure 3 Knockdown of JMJD1C does not affect IR-induced foci formation by RNF168, MDC1 and phosphorylated ATM.
(a-c) U2OS cells were transfected with siRNA oligonucleotides targeting the indicated molecules, incubated for 72 h, and exposed to IR (3Gy). (a) Two hours later cells were coimmunostained with antibodies to FK2 and γH2AX; Depletion of more than 85% and 95%, for JMJD1A and JMJD1B, respectively, was confirmed by realtime RT-PCR in the right panel. The values were normalized to β-actin mRNA and relative mRNA levels were calculated with ΔΔCt-method using control cells. (b) Two hours later cells were fixed by methanol and coimmunostained with antibodies to RNF168 and 53BP1. (c) Two hours later cells were coimmunostained with antibodies to MDC1 and phosphorylated ATM (Ser1981).
Supplementary Figure 4 Knockdown of JMJD1C affects neither global nor local histone modifications after DNA damage.
(a) U2OS cells were transfected with siRNA oligonucleotides targeting control or JMJD1C and incubated for 72 h. At indicated time points after exposure to IR (10Gy) various forms of histone modification in chromatin enriched fraction were analyzed by immunoblotting with the indicated antibodies. (b) Schematic representation of luciferase expression vector containing an I-SceI consensus cut site at 157 bp upstream of its promoter. The positions of the primers, which are spanning I-SceI consensus cut site and used in c, are indicated. (c) Genomic DNA was collected from one of these cell lines and PCR was performed using the primers as indicated in b. PCR amplicons were incubated with I-SceI endonuclease at 37 °C for 1 h and analyzed by agarose gel electrophoresis. Luciferase expressing vector containing an I-SceI consensus cut site was used for positive control. (d) Expression of luciferase in this cell line was monitored by luminometer. (e) Schematic representation of I-SceI consensus cut site and the positions of the primers used for ChIP analysis are indicated. (f and g) U2OS cells stably transfected with luciferase expression vector containing an I-SceI consensus cut site were transfected with siRNA oligonucleotides targeting control or JMJD1C, the next day transfected with mock or I-SceI expression vector, and after another 2 days cells were used for ChIP analysis. (f) Enrichment of histone H3 K9 mono-, di-, and trimethylation and MRE11 around DNA break site, respectively were analyzed using primers at 400bp downstream of I-SceI consensus cut site. The insertion of I-SceI consensus cut site was confirmed by PCR followed by I-SceI digestion in b and c and the expression of luciferase was monitored by luminometer in d. (g) Enrichment of histone H3 K9 dimethylation and MRE11 around the DNA break site was analyzed using primers at 400 bp, 500 bp and 1000 bp downstream of I-SceI consensus cut site. For negative control, primers at SAT2 loci were used.
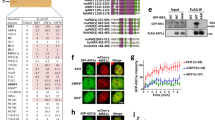
Supplementary Figure 5 JMJD1C interaction with MDC1, knockdown efficiencies of endogenous MDC1 and the expression of ‘siRNA-insensitive’ MDC1 proteins.
(a) U2OS cell lines stably expressing Flag-mock or Flag-JMJD1C were exposed or not to IR (5Gy) as indicated and crosslinked with DTBP. Cell nuclei were collected and dissolved by sonication in 1% SDS lysis buffer. Lysates were diluted to 0.1% SDS and subjected to immunoprecipitation with anti-Flag agarose followed by immunoblotting with antibodies to MDC1 and JMJD1C. (b) U2OS cells were transfected with siRNA oligonucleotides targeting control or MDC1, the next day transfected with siRNA-insesitive HA-tagged MDC1 (WT or K45A), and after another 2 days, the knockdown efficiencies of endogenous MDC1 and the expression of siRNA insensitive MDC1 proteins were monitored by immunoblotting with antibodies to MDC1 and HA.
Supplementary Figure 6 Tandem mass spectrometry (MS/MS) spectra for MDC1 peptides with lysine dimethylation.
Lysine di-methylation was identified at Lys-45, Lys-1075, Lys-1104, Lys-1140, Lys-1345 and Lys-1740 (see methods for details).
Supplementary Figure 7 Detection of endogenous JMJD1C and specificity validation for our DCS-410 antibody, and impact of JMJD1C knockdown on RAD51 focus formation in BRCA1-depleted cells.
(a and b) U2OS cell cells were transfected with siRNA oligonucleotides against control or JMJD1C, incubated for 72 h, and endogenous JMJD1C was detected by mouse DCS-410 antibody: (a) By immunofluorescence, JMJD1C showed a nuclear punctate staining pattern; Right panels show localization of stably expressed Flag-tagged JMJD1C in U2OS cells using anti-Flag antibody. (b) Our DCS-410 antibody against JMJD1C was superior to two commercial antibodies (from Bethyl and Abcam, respectively, the latter only recognizing a faster migrating non-specific band), in specifically detecting endogenous JMJD1C by immunoblotting,. (c) Immunobloting results with the DCS-410 antibody to JMJD1C on whole-cell lysates of two human breast cancer cell lines (SUM149 and MDA-MB-436) and U2OS cells. (d and e) U2OS cells were transfected with indicated siRNA oligonucleotides, incubated for 72 h, and knockdown efficiencies were analyzed by immunoblotting with the indicated antibodies (d), or (e): after exposure to IR (5Gy), cells were coimmunostained with antibodies to RAD51 and cyclin A; Representative immunofluorescence images of cells 6h after IR are shown.
Supplementary Figure 8 Uncropped images for the blots shown in the main figures.
(a) Anti–JMJD1C immunoblot of lysates of U2OS, HeLa cells and Human skin fibroblasts, shown in Fig. 1a. (b) Anti–JMJD1C immunoblot of lysates of U2OS cells treated with DMSO or 5 μM MG132 for 3h, shown in Fig. 1b. (c) JMJD1C fragment B interacting with GST-RNF8 and GST-RNF168 using GST pull-down assays, shown in Fig. 1c. (d) Anti-RAP80 and BRCA1 immunoblot of chromatin-enriched fractions from U2OS cells transfected with control or JMJD1C-targetting siRNAs at indicated times IR (10Gy), shown in Fig. 3e. (e) Anti–MDC1 and RNF8 immunoblot of immunoprecipitation of Flag-tagged RNF8 in U2OS cells treated with indicated siRNAs, exposed or not to IR (10Gy), shown in Fig. 4b. (f) Anti–RPA (phospho-S4S8 and total) immunoblot of U2OS cells (transfected with indicated siRNAs) at indicated times IR (5Gy), shown in Fig. 6a.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 and Supplementary Table 1 (PDF 1572 kb)
Rights and permissions
About this article
Cite this article
Watanabe, S., Watanabe, K., Akimov, V. et al. JMJD1C demethylates MDC1 to regulate the RNF8 and BRCA1–mediated chromatin response to DNA breaks. Nat Struct Mol Biol 20, 1425–1433 (2013). https://doi.org/10.1038/nsmb.2702
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.2702
This article is cited by
-
Reactivating antitumor immunity by inhibiting JMJD1C
Nature Immunology (2024)
-
A functional reference map of the RNF8 interactome in cancer
Biology Direct (2022)
-
Jmjd1c demethylates STAT3 to restrain plasma cell differentiation and rheumatoid arthritis
Nature Immunology (2022)
-
Histone H3 lysine 27 acetylation profile undergoes two global shifts in undernourished children and suggests altered one-carbon metabolism
Clinical Epigenetics (2021)
-
Circular RNA circ_0006168 enhances Taxol resistance in esophageal squamous cell carcinoma by regulating miR-194-5p/JMJD1C axis
Cancer Cell International (2021)