Abstract
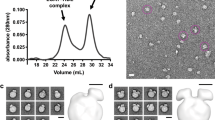
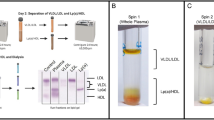
High-density lipoproteins (HDLs) mediate cholesterol transport and protection from cardiovascular disease. Although synthetic HDLs have been studied for 30 years, the structures of human plasma–derived HDL and its major protein apolipoprotein apoA-I are unknown. We separated normal human HDL into five density subfractions and then further isolated those containing predominantly apoA-I (LpA-I). Using cross-linking chemistry and mass spectrometry, we found that apoA-I adopts a structural framework in these particles that closely mirrors that in synthetic HDL. We adapted established structures for synthetic HDL to generate the first detailed models of authentic human plasma HDL in which apoA-I adopts a symmetrical cage-like structure. The models suggest that HDL particle size is modulated by means of a twisting motion of the resident apoA-I molecules. This understanding offers insights into how apoA-I structure modulates HDL function and its interactions with other apolipoproteins.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Gordon, T., Castelli, W.P., Hjortland, M.C., Kannel, W.B. & Dawber, T.R. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am. J. Med. 62, 707–714 (1977).
Rye, K.A., Bursill, C.A., Lambert, G., Tabet, F. & Barter, P.J. The metabolism and anti-atherogenic properties of HDL. J. Lipid Res. 50 Suppl, S195–S200 (2009).
Davidson, W.S. & Thompson, T.B. The structure of apolipoprotein A-I in high density lipoproteins. J. Biol. Chem. 282, 22249–22253 (2007).
Thomas, M.J., Bhat, S. & Sorci-Thomas, M.G. Three-dimensional models of HDL apoA-I: implications for its assembly and function. J. Lipid Res. 49, 1875–1883 (2008).
Segrest, J.P., Harvey, S.C. & Zannis, V. Detailed molecular model of apolipoprotein A-I on the surface of high-density lipoproteins and its functional implications. Trends Cardiovasc. Med. 10, 246–252 (2000).
Koppaka, V., Silvestro, L., Engler, J.A., Brouillette, C.G. & Axelsen, P.H. The structure of human lipoprotein A-I. Evidence for the 'belt' model. J. Biol. Chem. 274, 14541–14544 (1999).
Bhat, S., Sorci-Thomas, M.G., Alexander, E.T., Samuel, M.P. & Thomas, M.J. Intermolecular contact between globular N-terminal fold and C-terminal domain of ApoA-I stabilizes its lipid-bound conformation: studies employing chemical cross-linking and mass spectrometry. J. Biol. Chem. 280, 33015–33025 (2005).
Davidson, W.S. & Hilliard, G.M. The spatial organization of apolipoprotein A-I on the edge of discoidal high density lipoprotein particles: A mass spectrometry study. J. Biol. Chem. 278, 27199–27207 (2003).
Panagotopulos, S.E., Horace, E.M., Maiorano, J.N. & Davidson, W.S. Apoliprotein A-I adopts a belt-like orientation in reconstituted high density lipoproteins. J. Biol. Chem. 276, 42965–42970 (2001).
Silva, R.A., Hilliard, G.M., Li, L., Segrest, J.P. & Davidson, W.S. A mass spectrometric determination of the conformation of dimeric apolipoprotein A-I in discoidal high density lipoproteins. Biochemistry 44, 8600–8607 (2005).
Bhat, S., Sorci-Thomas, M.G., Tuladhar, R., Samuel, M.P. & Thomas, M.J. Conformational adaptation of apolipoprotein A-I to discretely sized phospholipid complexes. Biochemistry 46, 7811–7821 (2007).
Martin, D.D., Budamagunta, M.S., Ryan, R.O., Voss, J.C. & Oda, M.N. Apolipoprotein A-I assumes a 'looped belt' conformation on reconstituted high density lipoprotein. J. Biol. Chem. 281, 20418–20426 (2006).
Wu, Z. et al. The refined structure of nascent HDL reveals a key functional domain for particle maturation and dysfunction. Nat. Struct. Mol. Biol. 14, 861–868 (2007).
Wu, Z. et al. Double superhelix model of high density lipoprotein. J. Biol. Chem. 284, 36605–36619 (2009).
Gogonea, V. et al. Congruency between biophysical data from multiple platforms and molecular dynamics simulation of the double-super helix model of nascent high-density lipoprotein. Biochemistry 49, 7323–7343 (2010).
Jones, M.K. et al. Assessment of the validity of the double superhelix model for reconstituted high density lipoproteins: a combined computational-experimental approach. J. Biol. Chem. 285, 41161–41171 (2010).
Jonas, A., Wald, J.H., Toohill, K.L., Krul, E.S. & Kezdy, K.E. Apolipoprotein A-I structure and lipid properties in homogeneous, reconstituted spherical and discoidal high density lipoproteins. J. Biol. Chem. 265, 22123–22129 (1990).
Segrest, J.P., Garber, D.W., Brouillette, C.G., Harvey, S.C. & Anantharamaiah, G.M. The amphipathic alpha helix: a multifunctional structural motif in plasma apolipoproteins. Adv. Protein Chem. 45, 303–369 (1994).
Li, H.H. et al. ApoA-I structure on discs and spheres. Variable helix registry and conformational states. J. Biol. Chem. 277, 39093–39101 (2002).
Sparks, D.L., Phillips, M.C. & Lund-Katz, S. The conformation of apolipoprotein A-I in discoidal and spherical recombinant high density lipoprotein particles. 13C NMR studies of lysine ionization behavior. J. Biol. Chem. 267, 25830–25838 (1992).
Silva, R.A. et al. Structure of apolipoprotein A-I in spherical high density lipoproteins of different sizes. Proc. Natl. Acad. Sci. USA 105, 12176–12181 (2008).
Catte, A. et al. Novel changes in discoidal high density lipoprotein morphology: a molecular dynamics study. Biophys. J. 90, 4345–4360 (2006).
Gu, F. et al. Structures of discoidal high density lipoproteins: a combined computational-experimental approach. J. Biol. Chem. 285, 4652–4665 (2010).
Kontush, A., Chantepie, S. & Chapman, M.J. Small, dense HDL particles exert potent protection of atherogenic LDL against oxidative stress. Arterioscler. Thromb. Vasc. Biol. 23, 1881–1888 (2003).
Rosales, C., Gillard, B.K., Courtney, H.S., Blanco-Vaca, F. & Pownall, H.J. Apolipoprotein modulation of streptococcal serum opacity factor activity against human plasma high-density lipoproteins. Biochemistry 48, 8070–8076 (2009).
Kontush, A. et al. Preferential sphingosine-1-phosphate enrichment and sphingomyelin depletion are key features of small dense HDL3 particles: relevance to antiapoptotic and antioxidative activities. Arterioscler. Thromb. Vasc. Biol. 27, 1843–1849 (2007).
Davidson, W.S. et al. Proteomic analysis of defined HDL subpopulations reveals particle-specific protein clusters: relevance to antioxidative function. Arterioscler. Thromb. Vasc. Biol. 29, 870–876 (2009).
Brouillette, C.G. & Anantharamaiah, G.M. Structural models of human apolipoprotein A-I. Biochim. Biophys. Acta 1256, 103–129 (1995).
de Souza, J.A. et al. Metabolic syndrome features small, apolipoprotein A-I-poor, triglyceride-rich HDL3 particles with defective anti-apoptotic activity. Atherosclerosis 197, 84–94 (2008).
Ibdah, J.A., Lund-Katz, S. & Phillips, M.C. Molecular packing of high-density and low-density lipoprotein surface lipids and apolipoprotein A-I binding. Biochemistry 28, 1126–1133 (1989).
Hofsäss, C., Lindahl, E. & Edholm, O. Molecular dynamics simulations of phospholipid bilayers with cholesterol. Biophys. J. 84, 2192–2206 (2003).
Gordon, S.M., Deng, J., Lu, L.J. & Davidson, W.S. Proteomic characterization of human plasma high density lipoprotein fractionated by gel filtration chromatography. J. Proteome Res. 9, 5239–5249 (2010).
Curtiss, L.K., Bonnet, D.J. & Rye, K.A. The conformation of apolipoprotein A-I in high-density lipoproteins is influenced by core lipid composition and particle size: a surface plasmon resonance study. Biochemistry 39, 5712–5721 (2000).
Córsico, B., Toledo, J.D. & Garda, H.A. Evidence for a central apolipoprotein A-I domain loosely bound to lipids in discoidal lipoproteins that is capable of penetrating the bilayer of phospholipid vesicles. J. Biol. Chem. 276, 16978–16985 (2001).
Maiorano, J.N., Jandacek, R.J., Horace, E.M. & Davidson, W.S. Identification and structural ramifications of a hinge domain in apolipoprotein A-I discoidal high-density lipoproteins of different size. Biochemistry 43, 11717–11726 (2004).
Jones, M.K., Catte, A., Li, L. & Segrest, J.P. Dynamics of activation of lecithin:cholesterol acyltransferase by apolipoprotein A-I. Biochemistry 48, 11196–11210 (2009).
Roberts, L.M. et al. Structural analysis of apolipoprotein A-I: limited proteolysis of methionine-reduced and -oxidized lipid-free and lipid-bound human apo A-I. Biochemistry 36, 7615–7624 (1997).
Catte, A. et al. Structure of spheroidal HDL particles revealed by combined atomistic and coarse-grained simulations. Biophys. J. 94, 2306–2319 (2008).
Borhani, D.W., Rogers, D.P., Engler, J.A. & Brouillette, C.G. Crystal structure of truncated human apolipoprotein A-I suggests a lipid-bound conformation. Proc. Natl. Acad. Sci. USA 94, 12291–12296 (1997).
Lund-Katz, S. et al. Surface plasmon resonance analysis of the mechanism of binding of apoA-I to high density lipoprotein particles. J. Lipid Res. 51, 606–617 (2010).
Vaisar, T. et al. Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. J. Clin. Invest. 117, 746–756 (2007).
Chapman, M.J., Goldstein, S., Lagrange, D. & Laplaud, P.M. A density gradient ultracentrifugal procedure for the isolation of the major lipoprotein classes from human serum. J. Lipid Res. 22, 339–358 (1981).
Zerrad-Saadi, A. et al. HDL3-mediated inactivation of LDL-associated phospholipid hydroperoxides is determined by the redox status of apolipoprotein A-I and HDL particle surface lipid rigidity: relevance to inflammation and atherogenesis. Arterioscler. Thromb. Vasc. Biol. 29, 2169–2175 (2009).
Markwell, M.A., Haas, S.M., Bieber, L.L. & Tolbert, N.E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal. Biochem. 87, 206–210 (1978).
Acknowledgements
This work was supported by US National Institutes of Health (NIH) 01 grant HL67093 (to W.S.D.), an American Heart Association Great Rivers Postdoctoral Fellowship to R.H. (3880030), NIH R01 grant HL48148 (to W.G.J.) and NIH Pathway to Independence Award (K99/R00) HL087561 from the National Heart, Lung, and Blood Institute (to R.A.G.D.S.). Negative stain EM was carried out in the Vanderbilt University Research Electron Microscopy Resource of the Cell Imaging Core. This resource is partially supported by NIH grants CA68485, DK20593 and DK58404. A.K. was supported by Institut National de la Santé et de la Recherche Médicale, Fondation pour la Recherche Médicale en France. The images in Figure 6 were rendered by M. Hartsock (marcia@hartsockillustration.com). Any use of these images is subject to copyright law and should be negotiated with the artist. We also thank C. Smith for excellent administrative assistance.
Author information
Authors and Affiliations
Contributions
R.H., M.J.C. and W.S.D. designed the research plan. R.H., R.A.G.D.S., L.K.C., W.G.J. and A.K. conducted experiments. R.H., W.S.D. and T.J.H. analyzed data, and R.H. and W.S.D. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7, Supplementary Tables 1 and 2, and Supplementary Methods (PDF 1047 kb)
Rights and permissions
About this article
Cite this article
Huang, R., Silva, R., Jerome, W. et al. Apolipoprotein A-I structural organization in high-density lipoproteins isolated from human plasma. Nat Struct Mol Biol 18, 416–422 (2011). https://doi.org/10.1038/nsmb.2028
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.2028
This article is cited by
-
Quantifying PON1 on HDL with nanoparticle-gated electrokinetic membrane sensor for accurate cardiovascular risk assessment
Nature Communications (2023)
-
Size matters: HDL particle populations and the risk of infection
Nature Reviews Cardiology (2023)
-
High-density lipoproteins, reverse cholesterol transport and atherogenesis
Nature Reviews Cardiology (2021)
-
Structure–function analysis of naturally occurring apolipoprotein A-I L144R, A164S and L178P mutants provides insight on their role on HDL levels and cardiovascular risk
Cellular and Molecular Life Sciences (2021)