Abstract
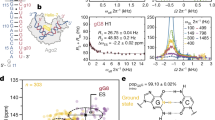
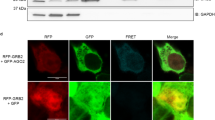
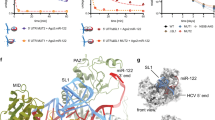
Small interfering RNAs (siRNAs) and microRNAs (miRNAs) bind to Argonaute (AGO) family proteins to form a related set of effector complexes that have diverse roles in post-transcriptional gene regulation throughout the eukaryotic lineage. Here sequence and structural analysis of the MID domain of the AGO proteins identified similarities with a family of allosterically regulated bacterial ligand-binding domains. We used in vitro and in vivo approaches to show that certain AGO proteins (those involved in translational repression) have conserved this functional allostery between two distinct sites, one involved in binding miRNA–target duplex and the other in binding the 5′ cap feature (m7GpppG) of eukaryotic mRNAs. This allostery provides an explanation for how miRNA-bound effector complexes may avoid indiscriminate repressive action (mediated through binding interactions with the cap) before full target recognition.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Ghildiyal, M. & Zamore, P.D. Small silencing RNAs: an expanding universe. Nat. Rev. Genet. 10, 94–108 (2009).
Carthew, R.W. & Sontheimer, E.J. Origins and mechanisms of miRNAs and siRNAs. Cell 136, 642–655 (2009).
Wu, L. & Belasco, J.G. Let me count the ways: mechanisms of gene regulation by miRNAs and siRNAs. Mol. Cell 29, 1–7 (2008).
Kiriakidou, M. et al. An mRNA m7G cap binding–like motif within human AGO2 represses translation. Cell 129, 1141–1151 (2007).
Pillai, R.S. et al. Inhibition of translational initiation by Let-7 microRNA in human cells. Science 309, 1573–1576 (2005).
Humphreys, D.T., Westman, B.J., Martin, D.I. & Preiss, T. MicroRNAs control translation initiation by inhibiting eukaryotic initiation factor 4E/cap and poly(A) tail function. Proc. Natl. Acad. Sci. USA 102, 16961–16966 (2005).
Wakiyama, M., Takimoto, K., Ohara, O. & Yokoyama, S. Let-7 microRNA-mediated mRNA deadenylation and translational repression in a mammalian cell-free system. Genes Dev. 21, 1857–1862 (2007).
Zdanowicz, A. et al. Drosophila miR2 primarily targets the m7GpppN cap structure for translational repression. Mol. Cell 35, 881–888 (2009).
Wang, B., Love, T.M., Call, M.E., Doench, J.G. & Novina, C.D. Recapitulation of short RNA-directed translational gene silencing in vitro. Mol. Cell 22, 553–560 (2006).
Wang, B., Yanez, A. & Novina, C.D. MicroRNA-repressed mRNAs contain 40S but not 60S components. Proc. Natl. Acad. Sci. USA 105, 5343–5348 (2008).
Rehwinkel, J., Behm-Ansmant, I., Gatfield, D. & Izaurralde, E. A crucial role for GW182 and the DCP1: DCP2 decapping complex in miRNA-mediated gene silencing. RNA 11, 1640–1647 (2005).
Eulalio, A., Helms, S., Fritzsch, C., Fauser, M. & Izaurralde, E. A C-terminal silencing domain in GW182 is essential for miRNA function. RNA 15, 1067–1077 (2009).
Chekulaeva, M., Filipowicz, W. & Parker, R. Multiple independent domains of dGW182 function in miRNA-mediated repression in Drosophila. RNA 15, 794–803 (2009).
Soding, J., Biegert, A. & Lupas, A.N. The HHpred interactive server for protein homology detection and structure prediction. Nucleic Acids Res. 33, W244–W248 (2005).
Song, J.J., Smith, S.K., Hannon, G.J. & Joshua-Tor, L. Crystal structure of argonaute and its implications for RISC slicer activity. Science 305, 1434–1437 (2004).
Marcotrigiano, J., Gingras, A.C., Sonenberg, N. & Burley, S.K. Cocrystal structure of the messenger RNA 5′ cap–binding protein (eIF4E) bound to 7-methyl-GDP. Cell 89, 951–961 (1997).
Holm, L. & Sander, C. Protein-structure comparison by alignment of distance matrices. J. Mol. Biol. 233, 123–138 (1993).
Anantharaman, V. & Aravind, L. Diversification of catalytic activities and ligand interactions in the protein fold shared by the sugar isomerases, elF2B, DeoR transcription factors Acyl-CoA transferases and methenyltetrahydrofolate synthetase. J. Mol. Biol. 356, 823–842 (2006).
Frickey, T. & Lupas, A. CLANS: a Java application for visualizing protein families based on pairwise similarity. Bioinformatics 20, 3702–3704 (2004).
Carmell, M.A., Xuan, Z., Zhang, M.Q. & Hannon, G.J. The Argonaute family: tentacles that reach into RNAi, developmental control, stem cell maintenance, and tumorigenesis. Genes Dev. 16, 2733–2742 (2002).
Parker, J.S. & Barford, D. Argonaute: a scaffold for the function of short regulatory RNAs. Trends Biochem. Sci. 31, 622–630 (2006).
Mi, S. et al. Sorting of small RNAs into Arabidopsis argonaute complexes is directed by the 5′ terminal nucleotide. Cell 133, 116–127 (2008).
Verdel, A. et al. RNAi-mediated targeting of heterochromatin by the RITS complex. Science 303, 672–676 (2004).
Meister, G. et al. Identification of novel argonaute-associated proteins. Curr. Biol. 15, 2149–2155 (2005).
Till, S. et al. A conserved motif in Argonaute-interacting proteins mediates functional interactions through the Argonaute PIWI domain. Nat. Struct. Mol. Biol. 14, 897–903 (2007).
Ding, L., Spencer, A., Morita, K. & Han, M. The developmental timing regulator AIN-1 interacts with miRISCs and may target the argonaute protein ALG-1 to cytoplasmic P bodies in C. elegans. Mol. Cell 19, 437–447 (2005).
Schumacher, M.A., Choi, K.Y., Zalkin, H. & Brennan, R.G. Crystal structure of LacI member, PurR, bound to DNA: minor groove binding by α helices. Science 266, 763–770 (1994).
Ma, J.B. et al. Structural basis for 5′-end–specific recognition of guide RNA by the A. fulgidus Piwi protein. Nature 434, 666–670 (2005).
Parker, J.S., Roe, S.M. & Barford, D. Structural insights into mRNA recognition from a PIWI domain–siRNA guide complex. Nature 434, 663–666 (2005).
Eulalio, A., Huntzinger, E. & Izaurralde, E. GW182 interaction with Argonaute is essential for miRNA-mediated translational repression and mRNA decay. Nat. Struct. Mol. Biol. 15, 346–353 (2008).
Lagos-Quintana, M., Rauhut, R., Lendeckel, W. & Tuschl, T. Identification of novel genes coding for small expressed RNAs. Science 294, 853–858 (2001).
Pillai, R.S., Artus, C.G. & Filipowicz, W. Tethering of human AGO proteins to mRNA mimics the miRNA-mediated repression of protein synthesis. Rna 10, 1518–1525 (2004).
Rehwinkel, J. et al. Genome-wide analysis of mRNAs regulated by Drosha and Argonaute proteins in Drosophila melanogaster. Mol. Cell. Biol. 26, 2965–2975 (2006).
Okamura, K., Ishizuka, A., Siomi, H. & Siomi, M.C. Distinct roles for argonaute proteins in small RNA–directed RNA cleavage pathways. Genes Dev. 18, 1655–1666 (2004).
Kinch, L.N. & Grishin, N.V. The human AGO2 MC region does not contain an eIF4E-like mRNA cap binding motif. Biol. Direct 4, 2 (2009).
Mathonnet, G. et al. MicroRNA inhibition of translation initiation in vitro by targeting the cap-binding complex eIF4F. Science 317, 1764–1767 (2007).
Miyoshi, K., Okada, T.N., Siomi, H. & Siomi, M.C. Characterization of the miRNA-RISC loading complex and miRNA-RISC formed in the Drosophila miRNA pathway. RNA 15, 1282–1291 (2009).
Miyoshi, K., Tsukumo, H., Nagami, T., Siomi, H. & Siomi, M.C. Slicer function of Drosophila Argonautes and its involvement in RISC formation. Genes Dev. 19, 2837–2848 (2005).
Tomari, Y., Du, T. & Zamore, P.D. Sorting of Drosophila small silencing RNAs. Cell 130, 299–308 (2007).
Vasudevan, S., Tong, Y. & Steitz, J.A. Cell-cycle control of microRNA-mediated translation regulation. Cell Cycle 7, 1545–1549 (2008).
Tu, B.P. et al. Cyclic changes in metabolic state during the life of a yeast cell. Proc. Natl. Acad. Sci. USA 104, 16886–16891 (2007).
Liu, J. et al. A role for the P-body component GW182 in microRNA function. Nat. Cell Biol. 7, 1261–1266 (2005).
Liu, J., Valencia-Sanchez, M.A., Hannon, G.J. & Parker, R. MicroRNA-dependent localization of targeted mRNAs to mammalian P-bodies. Nat. Cell Biol. 7, 719–723 (2005).
Pauley, K.M. et al. Formation of GW bodies is a consequence of microRNA genesis. EMBO Rep. 7, 904–910 (2006).
Turnbough, C.L., Jr. & Switzer, R.L. Regulation of pyrimidine biosynthetic gene expression in bacteria: repression without repressors. Microbiol. Mol. Biol. Rev. 72, 266–300 (2008).
Wang, Y.L. et al. Structure of an argonaute silencing complex with a seed-containing guide DNA and target RNA duplex. Nature 456, 921–926 (2008).
Wang, Y.L., Sheng, G., Juranek, S., Tuschl, T. & Patel, D.J. Structure of the guide-strand-containing argonaute silencing complex. Nature 456, 209–213 (2008).
Wang, Y. et al. Nucleation, propagation and cleavage of target RNAs in AGO silencing complexes. Nature 461, 754–761 (2009).
Guang, S. et al. An Argonaute transports siRNAs from the cytoplasm to the nucleus. Science 321, 537–541 (2008).
Ginalski, K., Elofsson, A., Fischer, D. & Rychlewski, L. 3D-Jury: a simple approach to improve protein structure predictions. Bioinformatics 19, 1015–1018 (2003).
Thompson, J.D., Higgins, D.G. & Gibson, T.J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673–4680 (1994).
Arnold, K., Bordoli, L., Kopp, J. & Schwede, T. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics 22, 195–201 (2006).
Miyoshi, K., Uejima, H., Nagami-Okada, T., Siomi, H. & Siomi, M.C. In vitro RNA cleavage assay for Argonaute-family proteins. Methods Mol. Biol. 442, 29–43 (2008).
Nahvi, A., Shoemaker, C.J. & Green, R. An expanded seed sequence definition accounts for full regulation of the hid 3′ UTR by bantam miRNA. RNA 15, 814–822 (2009).
Cong, P.J. & Shuman, S. Mutational analysis of messenger RNA capping enzyme identifies amino acids involved in GTP binding, enzyme-guanylate formation, and GMP transfer to RNA. Mol. Cell. Biol. 15, 6222–6231 (1995).
Stockley, P.G. Filter-binding assays. Methods Mol. Biol. 543, 1–14 (2009).
O'Hara, B.P. et al. Crystal structure and induction mechanism of AmiC–AmiR: a ligand-regulated transcription antitermination complex. EMBO J. 18, 5175–5186 (1999).
Acknowledgements
We thank S. Dorner for early contributions to the project, E. Izaurralde (Max Planck Institute) for providing the luciferase reporter constructs, H. Zaher (Johns Hopkins Univ. School of Medicine) for mRNA constructs used in filter binding assays, and J. Mendell, G. Seydoux, J. Lorsch, L. Cochella and H. Zaher for helpful comments on the manuscript. J.K.H. was supported by The Samsung Foundation of Culture. The project was supported by funding from the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
S.D. performed the bioinformatic analyses and in vitro studies; M.K.Z. performed the in vivo studies; J.K.H., A.N., J.L.B. and E.J.R. performed biochemistry in support of the main experiments; R.G. advised on the project; S.D., M.K.Z. and R.G. prepared the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 and Supplementary Methods (PDF 1899 kb)
Rights and permissions
About this article
Cite this article
Djuranovic, S., Zinchenko, M., Hur, J. et al. Allosteric regulation of Argonaute proteins by miRNAs. Nat Struct Mol Biol 17, 144–150 (2010). https://doi.org/10.1038/nsmb.1736
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.1736
This article is cited by
-
MicroRNAs recruit eIF4E2 to repress translation of target mRNAs
Protein & Cell (2017)
-
Molecular dissection of human Argonaute proteins by DNA shuffling
Nature Structural & Molecular Biology (2013)
-
The true core of RNA silencing revealed
Nature Structural & Molecular Biology (2012)
-
The mechanics of miRNA-mediated gene silencing: a look under the hood of miRISC
Nature Structural & Molecular Biology (2012)
-
Genome-wide identification of Ago2 binding sites from mouse embryonic stem cells with and without mature microRNAs
Nature Structural & Molecular Biology (2011)