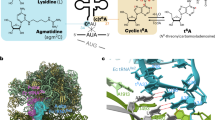
Abstract
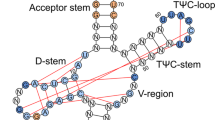
The bases at positions 32 and 38 in the tRNA anticodon loop are known to have a specific conservation depending upon the anticodon triplets. Here we report that evolutionarily conserved pairs of bases at positions 32 and 38 in tRNAAlaGGC prevent misreading of a near-cognate valine codon, GUC. The tRNAAlaGGC molecules with the conserved A32-U38 and C32-G38 pairs do not read GUC, whereas those with three representative nonconserved pairs, U32-U38, U32-A38 and C32-A38, direct the misincorporation of alanine at this valine codon into the peptide chain. Overexpression of the nonconserved tRNAAlaGGC in Escherichia coli is toxic and prevents cell growth. These results suggested that the bases at positions 32 and 38 in tRNAAlaGGC evolved to preserve the fidelity of the cognate codon reading.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Crick, F.H. Codon–anticodon pairing: the wobble hypothesis. J. Mol. Biol. 19, 548–555 (1966).
Calderon, I.L., Contopoulou, C.R. & Mortimer, R.K. Isolation of a DNA fragment that is expressed as an amber suppressor when present in high copy number in yeast. Gene 29, 69–76 (1984).
Pure, G.A., Robinson, G.W., Naumovski, L. & Friedberg, E.C. Partial suppression of an ochre mutation in Saccharomyces cerevisiae by multicopy plasmids containing a normal yeast tRNAGln gene. J. Mol. Biol. 183, 31–42 (1985).
Lin, J.P., Aker, M., Sitney, K.C. & Mortimer, R.K. First position wobble in codon-anticodon pairing: amber suppression by a yeast glutamine tRNA. Gene 49, 383–388 (1986).
Weiss, W.A. & Friedberg, E.C. Normal yeast tRNACAGGln can suppress amber codons and is encoded by an essential gene. J. Mol. Biol. 192, 725–735 (1986).
Weiss, W.A., Edelman, I., Culbertson, M.R. & Friedberg, E.C. Physiological levels of normal tRNACAGGln can effect partial suppression of amber mutations in the yeast Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 84, 8031–8034 (1987).
Toth, M.J., Murgola, E.J. & Schimmel, P. Evidence for a unique first position codon-anticodon mismatch in vivo. J. Mol. Biol. 201, 451–454 (1988).
Hirsh, D. Tryptophan transfer RNA as the UGA suppressor. J. Mol. Biol. 58, 439–458 (1971).
Cochella, L. & Green, R. An active role for tRNA in decoding beyond codon:anticodon pairing. Science 308, 1178–1180 (2005).
Schultz, D.W. & Yarus, M. tRNA structure and ribosomal function. I. tRNA nucleotide 27–43 mutations enhance first position wobble. J. Mol. Biol. 235, 1381–1394 (1994).
Schultz, D.W. & Yarus, M. tRNA structure and ribosomal function. II. Interaction between anticodon helix and other tRNA mutations. J. Mol. Biol. 235, 1395–1405 (1994).
Takai, K. & Yokoyama, S. Roles of 5-substituents of tRNA wobble uridines in the recognition of purine-ending codons. Nucleic Acids Res. 31, 6383–6391 (2003).
Agris, P.F., Vendeix, F.A. & Graham, W.D. tRNA's wobble decoding of the genome: 40 years of modification. J. Mol. Biol. 366, 1–13 (2007).
Lustig, F. et al. Codon discrimination and anticodon structural context. Proc. Natl. Acad. Sci. USA 86, 6873–6877 (1989).
Lustig, F. et al. The nucleotide in position 32 of the tRNA anticodon loop determines ability of anticodon UCC to discriminate among glycine codons. Proc. Natl. Acad. Sci. USA 90, 3343–3347 (1993).
Yarus, M. Translational efficiency of transfer RNA's: uses of an extended anticodon. Science 218, 646–652 (1982).
Raftery, L.A. & Yarus, M. Site-specific mutagenesis of Escherichia coli gltT yields a weak, glutamic acid-inserting ochre suppressor. J. Mol. Biol. 184, 343–345 (1985).
Yarus, M., Cline, S.W., Wier, P., Breeden, L. & Thompson, R.C. Actions of the anticodon arm in translation on the phenotypes of RNA mutants. J. Mol. Biol. 192, 235–255 (1986).
Raftery, L.A. & Yarus, M. Systematic alterations in the anticodon arm make tRNAGlu-Suoc a more efficient suppressor. EMBO J. 6, 1499–1506 (1987).
Smith, D., Breeden, L., Farrell, E. & Yarus, M. The bases of the tRNA anticodon loop are independent by genetic criteria. Nucleic Acids Res. 15, 4669–4686 (1987).
Olejniczak, M. & Uhlenbeck, O.C. tRNA residues that have coevolved with their anticodon to ensure uniform and accurate codon recognition. Biochimie 88, 943–950 (2006).
Olejniczak, M., Dale, T., Fahlman, R.P. & Uhlenbeck, O.C. Idiosyncratic tuning of tRNAs to achieve uniform ribosome binding. Nat. Struct. Mol. Biol. 12, 788–793 (2005).
Sprinzl, M., Horn, C., Brown, M., Ioudovitch, A. & Steinberg, S. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 26, 148–153 (1998).
Shimizu, Y. et al. Cell-free translation reconstituted with purified components. Nat. Biotechnol. 19, 751–755 (2001).
Hou, Y.M. & Schimmel, P. A simple structural feature is a major determinant of the identity of a transfer RNA. Nature 333, 140–145 (1988).
Francklyn, C. & Schimmel, P. Aminoacylation of RNA minihelices with alanine. Nature 337, 478–481 (1989).
Tamura, K., Asahara, H., Himeno, H., Hasegawa, T. & Shimizu, M. Identity elements of Escherichia coli tRNAAla. J. Mol. Recognit. 4, 129–132 (1991).
Normanly, J., Ogden, R.C., Horvath, S.J. & Abelson, J. Changing the identity of a transfer RNA. Nature 321, 213–219 (1986).
Asahara, H. et al. Recognition nucleotides of Escherichia coli tRNALeu and its elements facilitating discrimination from tRNASer and tRNATyr. J. Mol. Biol. 231, 219–229 (1993).
Tukalo, M., Yaremchuk, A., Fukunaga, R., Yokoyama, S. & Cusack, S. The crystal structure of leucyl-tRNA synthetase complexed with tRNALeu in the post-transfer-editing conformation. Nat. Struct. Mol. Biol. 12, 923–930 (2005).
Rodnina, M.V. & Wintermeyer, W. Fidelity of aminoacyl-tRNA selection on the ribosome: kinetic and structural mechanisms. Annu. Rev. Biochem. 70, 415–435 (2001).
Rodnina, M.V. & Wintermeyer, W. Ribosome fidelity: tRNA discrimination, proofreading and induced fit. Trends Biochem. Sci. 26, 124–130 (2001).
Pape, T., Wintermeyer, W. & Rodnina, M.V. Complete kinetic mechanism of elongation factor Tu-dependent binding of aminoacyl-tRNA to the A site of the E. coli ribosome. EMBO J. 17, 7490–7497 (1998).
Pape, T., Wintermeyer, W. & Rodnina, M. Induced fit in initial selection and proofreading of aminoacyl-tRNA on the ribosome. EMBO J. 18, 3800–3807 (1999).
Ledoux, S., Olejniczak, M. & Uhlenbeck, O.C. A sequence element that tunes Escherichia coli tRNAAlaGGC to ensure accurate decoding. Nat. Struct. Mol. Biol. advance online publication, doi:10.1038/nsmb.1581 (22 March 2009).
Auffinger, P. & Westhof, E. Singly and bifurcated hydrogen-bonded base-pairs in tRNA anticodon hairpins and ribozymes. J. Mol. Biol. 292, 467–483 (1999).
Saks, M.E. & Conery, J.S. Anticodon-dependent conservation of bacterial tRNA gene sequences. RNA 13, 651–660 (2007).
Milligan, J.F., Groebe, D.R., Witherell, G.W. & Uhlenbeck, O.C. Oligoribonucleotide synthesis using T7 RNA polymerase and synthetic DNA templates. Nucleic Acids Res. 15, 8783–8798 (1987).
Shimizu, Y., Kanamori, T. & Ueda, T. Protein synthesis by pure translation systems. Methods 36, 299–304 (2005).
Goto, Y. et al. Reprogramming the translation initiation for the synthesis of physiologically stable cyclic peptides. ACS Chem. Biol. 3, 120–129 (2008).
Kawakami, T., Murakami, H. & Suga, H. Messenger RNA-programmed incorporation of multiple N-methyl-amino acids into linear and cyclic peptides. Chem. Biol. 15, 32–42 (2008).
Sako, Y., Goto, Y., Murakami, H. & Suga, H. Ribosomal synthesis of peptidase-resistant peptides closed by a nonreducible inter-side-chain bond. ACS Chem. Biol. 3, 241–249 (2008).
Acknowledgements
We thank O.C. Uhlenbeck and S. Ledoux for their invaluable discussion. This work was supported by grants from the Japan Society for the Promotion of Science (JSPS) Grants-in-Aid for Scientific Research (S) (16101007) to H.S., a Young Scientists (A) (20681022) to H.M., a JSPS Fellowship (19-1722) to A.O., a research and development project of the Industrial Science and Technology Program in the New Energy and Industrial Technology Development Organization (NEDO) to H.S., the Industrial Technology Research Grant Program in NEDO (05A02513a) to H.M., and the Takeda Science Foundation.
Author information
Authors and Affiliations
Contributions
This study was designed by H.M., A.O. and H.S.; all of the experiments were performed by H.M.; the paper was written by H.M. and H.S.
Corresponding authors
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4 and Supplementary Methods (PDF 4508 kb)
Rights and permissions
About this article
Cite this article
Murakami, H., Ohta, A. & Suga, H. Bases in the anticodon loop of tRNAAlaGGC prevent misreading. Nat Struct Mol Biol 16, 353–358 (2009). https://doi.org/10.1038/nsmb.1580
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.1580
This article is cited by
-
Translation of non-standard codon nucleotides reveals minimal requirements for codon-anticodon interactions
Nature Communications (2018)
-
Maintenance of protein synthesis reading frame by EF-P and m1G37-tRNA
Nature Communications (2015)
-
Natural amino acids do not require their native tRNAs for efficient selection by the ribosome
Nature Chemical Biology (2009)
-
In Brief
Nature Reviews Genetics (2009)
-
A tipping point for mistranslation and disease
Nature Structural & Molecular Biology (2009)