Abstract
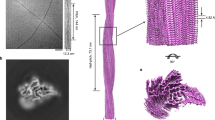
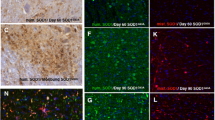
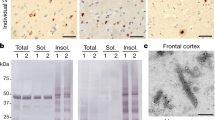
Mutations in the SOD1 gene cause the autosomal dominant, neurodegenerative disorder familial amyotrophic lateral sclerosis (FALS). In spinal cord neurons of human FALS patients and in transgenic mice expressing these mutant proteins, aggregates containing FALS SOD1 are observed. Accumulation of SOD1 aggregates is believed to interfere with axonal transport, protein degradation and anti-apoptotic functions of the neuronal cellular machinery. Here we show that metal-deficient, pathogenic SOD1 mutant proteins crystallize in three different crystal forms, all of which reveal higher-order assemblies of aligned β-sheets. Amyloid-like filaments and water-filled nanotubes arise through extensive interactions between loop and β-barrel elements of neighboring mutant SOD1 molecules. In all cases, non-native conformational changes permit a gain of interaction between dimers that leads to higher-order arrays. Normal β-sheet–containing proteins avoid such self-association by preventing their edge strands from making intermolecular interactions. Loss of this protection through conformational rearrangement in the metal-deficient enzyme could be a toxic property common to mutants of SOD1 linked to FALS.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Haverkamp, L.J., Appel, V. & Appel, S.H. Natural history of amyotrophic lateral sclerosis in a database population. Validation of a scoring system and a model for survival prediction. Brain 118, 707–719 (1995).
Deng, H.X. et al. Amyotrophic lateral sclerosis and structural defects in Cu,Zn superoxide dismutase. Science 261, 1047–1051 (1993).
Rosen, D.R. et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362, 59–62 (1993).
Fridovich, I. Superoxide dismutases. An adaptation to a paramagnetic gas. J. Biol. Chem. 264, 7761–7764 (1989).
Bowling, A.C., Schulz, J.B., Brown, R.H. Jr. & Beal, M.F. Superoxide dismutase activity, oxidative damage, and mitochondrial energy metabolism in familial and sporadic amyotrophic lateral sclerosis. J. Neurochem. 61, 2322–2325 (1993).
Reaume, A.G. et al. Motor neurons in Cu/Zn superoxide dismutase-deficient mice develop normally but exhibit enhanced cell death after axonal injury. Nat. Genet. 13, 43–47 (1996).
Ripps, M.E., Huntley, G.W., Hof, P.R., Morrison, J.H. & Gordon, J.W. Transgenic mice expressing an altered murine superoxide dismutase gene provide an animal model of amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. USA 92, 689–693 (1995).
Bruijn, L.I. et al. ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron 18, 327–338 (1997).
Gurney, M.E. et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science 264, 1772–1775 (1994).
Wong, P.C. et al. An adverse property of a familial ALS-linked SOD1 mutation causes motor neuron disease characterized by vacuolar degeneration of mitochondria. Neuron 14, 1105–1116 (1995).
Wiedau-Pazos, M. et al. Altered reactivity of superoxide dismutase in familial amyotrophic lateral sclerosis. Science 271, 515–518 (1996).
Yim, M.B. et al. A gain-of-function of an amyotrophic lateral sclerosis-associated Cu,Zn-superoxide dismutase mutant: an enhancement of free radical formation due to a decrease in Km for hydrogen peroxide. Proc. Natl. Acad. Sci. USA 93, 5709–5714 (1996).
Beckman, J.S., Chen, J., Crow, J.P. & Ye, Y.Z. Reactions of nitric oxide, superoxide and peroxynitrite with superoxide dismutase in neurodegeneration. Prog. Brain Res. 103, 371–380 (1994).
Estevez, A.G. et al. Induction of nitric oxide-dependent apoptosis in motor neurons by zinc-deficient superoxide dismutase. Science 286, 2498–2500 (1999).
Bruijn, L.I. et al. Aggregation and motor neuron toxicity of an ALS-linked SOD1 mutant independent from wild-type SOD1. Science 281, 1851–1854 (1998).
Wang, J. et al. Fibrillar inclusions and motor neuron degeneration in transgenic mice expressing superoxide dismutase 1 with a disrupted copper-binding site. Neurobiol. Dis. 10, 128–138 (2002).
Bruening, W. et al. Up-regulation of protein chaperones preserves viability of cells expressing toxic Cu/Zn-superoxide dismutase mutants associated with amyotrophic lateral sclerosis. J. Neurochem. 72, 693–699 (1999).
Okado-Matsumoto, A. & Fridovich, I. Amyotrophic lateral sclerosis: a proposed mechanism. Proc. Natl. Acad. Sci. USA 99, 9010–9014 (2002).
Borchelt, D.R. et al. Axonal transport of mutant superoxide dismutase 1 and focal axonal abnormalities in the proximal axons of transgenic mice. Neurobiol. Dis. 5, 27–35 (1998).
Williamson, T.L. & Cleveland, D.W. Slowing of axonal transport is a very early event in the toxicity of ALS-linked SOD1 mutants to motor neurons. Nat. Neurosci. 2, 50–56 (1999).
Johnston, J.A., Dalton, M.J., Gurney, M.E. & Kopito, R.R. Formation of high molecular weight complexes of mutant Cu, Zn-superoxide dismutase in a mouse model for familial amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. USA 97, 12571–12576 (2000).
Hayward, L.J. et al. Decreased metallation and activity in subsets of mutant superoxide dismutases associated with familial amyotrophic lateral sclerosis. J. Biol. Chem. 277, 15923–15931 (2002).
Liu, H. et al. Copper2+ binding to the surface residue cysteine 111 of His46Arg human copper-zinc superoxide dismutase, a familial amyotrophic lateral sclerosis mutant. Biochemistry 39, 8125–8132 (2000).
Rodriguez, J.A. et al. Familial ALS-associated mutations decrease the thermal stability of distinctly metallated species of human copper-zinc superoxide dismutase. J. Biol. Chem. 277, 15932–15937 (2002).
Lyons, T.J. et al. Mutations in copper-zinc superoxide dismutase that cause amyotrophic lateral sclerosis alter the zinc binding site and the redox behavior of the protein. Proc. Natl. Acad. Sci. USA 93, 12240–12244 (1996).
Crow, J.P., Sampson, J.B., Zhuang, Y., Thompson, J.A. & Beckman, J.S. Decreased zinc affinity of amyotrophic lateral sclerosis-associated superoxide dismutase mutants leads to enhanced catalysis of tyrosine nitration by peroxynitrite. J. Neurochem. 69, 1936–1944 (1997).
Lyons, T.J. et al. The metal binding properties of the zinc site of yeast copper-zinc superoxide dismutase: implications for amyotrophic lateral sclerosis. J. Biol. Inorg. Chem. 5, 189–203 (2000).
Subramaniam, J.R. et al. Mutant SOD1 causes motor neuron disease independent of copper chaperone-mediated copper loading. Nat. Neurosci. 5, 301–307 (2002).
Serag, A.A., Altenbach, C., Gingery, M., Hubbell, W.L. & Yeates, T.O. Arrangement of subunits and ordering of β-strands in an amyloid sheet. Nat. Struct. Biol. 9, 734–739 (2002).
Connors, L.H., Richardson, A.M., Theberge, R. & Costello, C.E. Tabulation of transthyretin (TTR) variants as of 1/1/2000. Amyloid 7, 54–69 (2000).
Richardson, J.S. & Richardson, D.C. Natural β-sheet proteins use negative design to avoid edge-to-edge aggregation. Proc. Natl. Acad. Sci. USA 99, 2754–2759 (2002).
Watanabe, M. et al. Histological evidence of protein aggregation in mutant SOD1 transgenic mice and in amyotrophic lateral sclerosis neural tissues. Neurobiol. Dis. 8, 933–941 (2001).
Strange, R.W. et al. The structure of metal deficient wild type human Cu,Zn superoxide dismutase and its relevance to familial amyotrophic lateral sclerosis. J. Mol. Biol. 328, 877–891 (2003).
Perutz, M.F., Finch, J.T., Berriman, J. & Lesk, A. Amyloid fibers are water-filled nanotubes. Proc. Natl. Acad. Sci. USA 99, 5591–5595 (2002).
Lashuel, H.A., Hartley, D., Petre, B.M., Walz, T. & Lansbury, P.T. Jr. Neurodegenerative disease: amyloid pores from pathogenic mutations. Nature 418, 291 (2002).
Sherman, M.Y. & Goldberg, A.L. Cellular defenses against unfolded proteins: a cell biologist thinks about neurodegenerative diseases. Neuron 29, 15–32 (2001).
Shibata, N. et al. Intense superoxide dismutase-1 immunoreactivity in intracytoplasmic hyaline inclusions of familial amyotrophic lateral sclerosis with posterior column involvement. J. Neuropathol. Exp. Neurol. 55, 481–490 (1996).
Wang, J., Xu, G. & Borchelt, D.R. High molecular weight complexes of mutant superoxide dismutase 1: age-dependent and tissue-specific accumulation. Neurobiol. Dis. 9, 139–148 (2002).
Taylor, J.P., Hardy, J. & Fischbeck, K.H. Toxic proteins in neurodegenerative disease. Science 296, 1991–1995 (2002).
Goto, J.J. et al. Loss of in vitro metal ion binding specificity in mutant copper-zinc superoxide dismutases associated with familial amyotrophic lateral sclerosis. J. Biol. Chem. 275, 1007–1014 (2000).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 306–326 (1997).
Hart, P.J. et al. Subunit asymmetry in the three-dimensional structure of a human CuZnSOD mutant found in familial amyotrophic lateral sclerosis. Protein Sci. 7, 545–555 (1998).
Navaza, J. & Saludjian, P. AMoRe: an automated molecular replacement program package. Methods Enzymol. 276, 581–594 (1997).
Kissinger, C.R., Gehlhaar, D.K. & Fogel, D.B. Rapid automated molecular replacement by evolutionary search. Acta Crystallogr. D 55, 484–491 (1999).
Vagin, A.A. & Teplyakov, A. MOLREP: an automated program for molecular replacement. J. Appl. Crystallogr. 30, 1022–1025 (1997).
Murshudov, G.N., Vagin, A.A., Lebedev, A., Wilson, K.S. & Dodson, E.J. Efficient anisotropic refinement of macromolecular structures using FFT. Acta Crystallogr. D 55, 247–255 (1999).
Brunger, A.T. et al. Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D 54, 905–921 (1998).
Jones, T.A., Zou, J.Y., Cowan, S.W. & Kjeldgaard, M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A 47, 110–119 (1991).
Winn, M.D., Isupov, M.N. & Murshudov, G.N. Use of TLS parameters to model anisotropic displacements in macromolecular refinement. Acta Crystallogr. D 57, 122–133 (2001).
Hooft, R.W., Vriend, G., Sander, C. & Abola, E.E. Errors in protein structures. Nature 381, 272 (1996).
Laskowski, R.A., McArthur, M.W., Moss, D.S. & Thornton, J.M. PROCHECK: a program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 26, 283–291 (1993).
Cohen, G.E. ALIGN: a program to superimpose protein coordinates, accounting for insertions and deletions. J. Appl. Crystallogr. 30, 1160–1161 (1997).
Kraulis, P.J. MOLSCRIPT: a program to produce both detailed and schematic plots of protein structures. J. Appl. Crystallogr. 24, 946–950 (1991).
Esnouf, R.M. Further additions to MolScript version 1.4, including reading and contouring of electron-density maps. Acta Crystallogr. D 55, 938–940 (1999).
Read, R.J. Improved Fourier coefficients for maps using phases from partial structures with errors. Acta Crystallogr. A 42, 140–149 (1986).
Acknowledgements
We thank L. Flaks, and J. Berendzen for support at beamline X8-C at the NSLS, Brookhaven National Laboratory; D. Cascio and M. Hough for their interest and valuable discussions; S. Holloway for assistance with the illustrations and our colleagues who have offered comments during the preparation of this manuscript. This work was supported by the National Institutes of Health (L.J.H., J.S.V. and P.J.H.), the Robert A. Welch Foundation (P.J.H.), the ALS Association (L.J.H., J.S.V and P.J.H.), the MND Association (S.S.H.) and a predoctoral fellowship from the Association for the Advancement of Aging Research (J.S.E.). Funding from CCLRC and resources at Daresbury are also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Elam, J., Taylor, A., Strange, R. et al. Amyloid-like filaments and water-filled nanotubes formed by SOD1 mutant proteins linked to familial ALS. Nat Struct Mol Biol 10, 461–467 (2003). https://doi.org/10.1038/nsb935
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsb935
This article is cited by
-
Structural analysis of the overoxidized Cu/Zn-superoxide dismutase in ROS-induced ALS filament formation
Communications Biology (2022)
-
Modeling of mutant superoxide dismutase 1 octamers with cross-linked disulfide bonds
Journal of Molecular Modeling (2022)
-
Nucleation and kinetics of SOD1 aggregation in human cells for ALS1
Molecular and Cellular Biochemistry (2020)
-
Computational Investigation on Electrostatic Loop Mutants Instigating Destabilization and Aggregation on Human SOD1 Protein Causing Amyotrophic Lateral Sclerosis
The Protein Journal (2019)
-
Molecular mechanisms underlying the impact of mutations in SOD1 on its conformational properties associated with amyotrophic lateral sclerosis as revealed with molecular modelling
BMC Structural Biology (2018)