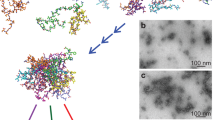
Abstract
Amyloid fibrils are associated with several disease states, but their structures have yet to be fully defined. Here we use site-directed spin labeling to explain some of the specific interactions that are formed between subunits when the protein transthyretin (TTR) assembles into amyloid fibrils, which are associated with both spontaneous and familial amyloid diseases in humans. The results suggest that fibrils are formed when a major conformational change displaces the terminal β-strand from the edge of a β-sheet in the native structure, exposing the penultimate strand. The newly exposed strand then allows a novel β-sheet interaction to form between the TTR subunits. This interaction and another previously identified subunit association lead to a plausible model for the specific sequence of β-strands in one of the indefinitely repeating β-sheets of TTR amyloid, which is formed by a head-to-head, tail-to-tail arrangement of subunits.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Sipe, J.D. Annu. Rev. Biochem. 61, 947–975 (1992).
Perutz, M.F. Curr. Opin. Struct. Biol. 6, 848–858 (1996).
Kelly, J.W. et al. Adv. Protein Chem. 50, 161–181 (1997).
Sunde, M. et al. J. Mol. Biol. 273, 729–739 (1997).
Westermark, P., Sletten, K., Johansson, B. & Cornwell, G.G. Proc. Natl. Acad. Sci. USA 87, 2843–2845 (1990).
Kelly, J.W. Curr. Opin. Struct. Biol. 8, 101–106 (1998).
Lai, Z., Colon, W. & Kelly, J.W. Biochemistry 35, 6470–6482 (1996).
Lashuel, H.A., Lai, Z. & Kelly, J.W. Biochemistry 37, 17851–17864 (1998).
Olofsson, A. et al. J. Biol. Chem. 276, 39592–39599 (2001).
Serpell, L.C. et al. J. Mol. Biol. 254, 113–118 (1995).
Serpell, L.C. et al. J Mol. Biol. 300, 1033–1039 (2000).
Blake, C.C. & Serpell, L.C. Structure 4, 989–998 (1996).
Serag, A.A., Altenbach, C., Gingery, M., Hubbell, W.L. & Yeates, T.O. Biochemistry 40, 9089–9096 (2001).
Serpell, L.C., Goldsteins, G., Dacklin, I., Lundgren, E. & Blake, C.C. Amyloid 3, 75–85 (1996).
Liu, K., Cho, H.S., Lashuel, H.A., Kelly, J.W. & Wemmer, D.E. Nature Struct. Biol. 7, 754–757 (2000).
Eneqvist, T., Andersson, K., Olofsson, A., Lundgren, E. & Sauer-Eriksson, A.E. Mol. Cell 6, 1207–1218 (2000).
Blake, C.C., Geisow, M.J., Oatley, S.J., Rerat, B. & Rerat, C. J. Mol. Biol. 121, 339–356 (1978).
Eneqvist, T. & Sauer-Eriksson, A.E. Amyloid 8, 149–168 (2001).
McParland, V.J., Kalverda, A.P., Homans, S.W. & Radford, S.E. Nature Struct. Biol. 9, 326–331 (2002).
Hoshino, M. et al. Nature Struct. Biol. 9, 332–336 (2002).
Liu, K. et al. J. Mol. Biol. 303, 555–655 (2000).
Ekiel, I. & Abrahamson, M. J. Biol. Chem. 271, 1314–1321 (1996).
Garzon-Rodriguez, W., Sepulveda-Becerra, M., Milton, S. & Glabe, C.G. J. Biol. Chem. 272, 21037–21044 (1997).
Friedhoff, P., Schneider, A., Mandelkow, E.M. & Mandelkow, E. Biochemistry 37, 10223–10230 (1998).
Enya, M. et al. Am. J. Pathol. 154, 271–279 (1999).
Liu, Y., Gotte, G., Libonati, M. & Eisenberg, D. Nature Struct. Biol. 8, 211–214 (2001).
Janowski, R. et al. Nature Struct. Biol. 8, 316–320 (2001).
Knaus, K.J. et al. Nature Struct. Biol. 8, 770–774 (2001).
Padilla, J.E., Colovos, C. & Yeates, T.O. Proc. Natl. Acad. Sci. USA 98, 2217–2221 (2001).
Sunde, M. & Blake, C. Adv. Protein Chem. 50, 123–159 (1997).
Rabenstein, M.D. & Shin, Y.K. Proc. Natl. Acad. Sci. USA 92, 8239–8243 (1995).
Altenbach, C., Oh, K.J., Trabanino, R.J., Hideg, K. & Hubbell, W.L. Biochemistry 40, 15471–15482 (2001).
Pake, G.E. J. Chem. Phys. 16, 327–336 (1948).
Gross, A., Columbus, L., Hideg, K., Altenbach, C. & Hubbell, W.L. Biochemistry 38, 10324–10335 (1999).
Richardson, J.S. & Richardson, D.C. Proc. Natl. Acad. Sci. USA 99, 2754–2759 (2002).
Carson, M. Methods Enzymol. 277, 493–505 (1997).
Acknowledgements
The authors thank L. Columbus, D. Eisenberg, M. Phillips and M. Sawaya for helpful discussions and technical expertise. This work was supported by the NIH and the DOE-BER program. A.A.S. was supported in part by the Medical Scientist Training Program of the UCLA School of Medicine.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Serag, A., Altenbach, C., Gingery, M. et al. Arrangement of subunits and ordering of β-strands in an amyloid sheet. Nat Struct Mol Biol 9, 734–739 (2002). https://doi.org/10.1038/nsb838
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsb838
This article is cited by
-
Considerably Unfolded Transthyretin Monomers Preceed and Exchange with Dynamically Structured Amyloid Protofibrils
Scientific Reports (2015)
-
A review on protein oligomerization process
International Journal of Precision Engineering and Manufacturing (2015)
-
High-resolution structure of infectious prion protein: the final frontier
Nature Structural & Molecular Biology (2012)
-
GroEL-induced topological dislocation of a substrate protein β-sheet core: a solution EPR spin–spin distance study
Journal of Chemical Biology (2010)
-
Amyloid formation by globular proteins under native conditions
Nature Chemical Biology (2009)