Abstract
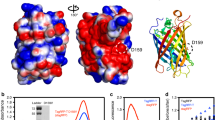
Green fluorescent protein (GFP) has rapidly become a standard tool for investigating a variety of cellular activities, and has served as a model system for understanding spectral tuning in chromophoric proteins. Distant homologs of GFP in reef coral and anemone display two new properties of the fluorescent protein family: dramatically red-shifted spectra, and oligomerization to form tetramers. We now report the 1.9 Å crystal structure of DsRed, a red fluorescent protein from Discosoma coral. DsRed monomers show similar topology to GFP, but additional chemical modification to the chromophore extends the conjugated π-system and likely accounts for the red-shifted spectra. Oligomerization of DsRed occurs at two chemically distinct protein interfaces to assemble the tetramer. The DsRed structure reveals the chemical basis for the functional properties of red fluorescent proteins and provides the basis for rational engineering of this subfamily of GFP homologs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Matz, M.V. et al. Nature Biotechnol. 17, 969–973 (1999).
Fradkov, A.F. et al. FEBS Lett. 479, 127–130 (2000).
Lukyanov, K.A. et al. J. Biol. Chem. 275, 25879–25882 (2000).
Prasher, D.C., Eckenrode, V.K., Ward, W.W., Prendergast, F.G. & Cormier, M.J. Gene 111, 229–233 (1992).
Tsien, R.Y. Annu. Rev. Biochem. 67, 509–544 (1998).
Heim, R., Prasher, D.C. & Tsien, R.Y. Proc. Natl. Acad. Sci. USA 91, 12501–12504 (1994).
Kneen, M., Farinas, J., Li, Y. & Verkman, A.S. Biophys. J. 74, 1591–1599 (1998).
Miyawaki, A. et al. Nature 388, 882–7 (1997).
Romoser, V.A., Hinkle, P.M. & Persechini, A. J. Biol. Chem. 272, 13270–12374 (1997).
Waldo, G.S., Standish, B.M., Berendzen, J. & Terwilliger, T.C. Nature Biotechnol. 17, 691–695 (1999).
Heim, R., Cubitt, A.B. & Tsien, R.Y. Nature 373, 663–664 (1995).
Chattoraj, M., King, B.A., Bublitz, G.U. & Boxer, S.G. Proc. Natl. Acad. Sci. USA 93, 8362–8367 (1996).
Ormö, M. et al. Science 273, 1392–1395 (1996).
Kroon, A.R. et al. J. Biol. Chem. 271, 31949–31956 (1996).
Kochendoerfer, G.G., Lin, S.W., Sakmar, T.P. & Mathies, R.A. Trends Biochem. Sci. 24, 300–305 (1999).
Cubitt, A.B., Woollenweber, L.A. & Heim, R. Methods Cell Biol. 58, 19–30 (1999).
Gross, L.A., Baird, G.S., Hoffman, R.C., Baldridge, K.K. & Tsien, R.Y. Proc. Natl. Acad. Sci. USA 97, 11990–11995 (2000).
Brunger, A.T. et al. Acta Crystallogr. D 54, 905–921 (1998).
Baird, G.S., Zacharias, D.A. & Tsien, R.Y. Proc. Natl. Acad. Sci. USA 97, 11984–11989 (2000).
Heikal, A.A., Hess, S.T., Baird, G.S., Tsien, R.Y. & Webb, W.W. Proc. Natl. Acad. Sci. USA 97, 11996–12001 (2000).
Bublitz, G., King, B.A. & Boxer, S.G. J. Am. Chem. Soc. 120, 9370–9371 (1998).
Cubitt, A.B. et al. Trends Biochem Sci 20, 448–455 (1995).
Reid, B.G. & Flynn, G.C. Biochemistry 36, 6786–6791 (1997).
Yang, F., Moss, L.G. & Phillips, G.N., Jr. Nature Biotechnol. 14, 1246–1251 (1996).
Drakenberg, T. & Forsén, S. J. Chem. Soc. Chem. Commun., 1404–1405 (1971).
Radzicka, A., Pedersen, L. & Wolfenden, R. Biochemistry 27, 4538–4541 (1988).
Jabs, A., Weiss, M.S. & Hilgenfeld, R. J. Mol. Biol. 286, 291–304 (1999).
Dominguez, R. et al. Nature Struct. Biol. 2, 569–576 (1995).
Perrakis, A. et al. Structure 2, 1169–1180 (1994).
Sakon, J., Adney, W.S., Himmel, M.E., Thomas, S.R. & Karplus, P.A. Biochemistry 35, 10648–10660 (1996).
Varghese, J.N. et al. Proc. Natl. Acad. Sci. USA 91, 2785–2789 (1994).
Hennig, M. et al. FEBS Lett 306, 80–84 (1992).
Wiesmann, C., Beste, G., Hengstenberg, W. & Schulz, G.E. Structure 3, 961–968 (1995).
Van Roey, P., Rao, V., Plummer, T.H., Jr. & Tarentino, A.L. Biochemistry 33, 13989–13996 (1994).
Terwisscha van Scheltinga, A.C., Hennig, M. & Dijkstra, B.W. J. Mol. Biol. 262, 243–257 (1996).
Perricaudet, M. & Pullman, A. Int. J. Pept. Protein Res. 5, 99–107 (1973).
Fersht, A.R. J. Am. Chem. Soc. 93, 3504–3515 (1971).
Haupts, U., Maiti, S., Schwille, P. & Webb, W.W. Proc. Natl. Acad. Sci. USA 95, 13573–13578 (1998).
Clackson, T. & Wells, J.A. Science 267, 383–386 (1995).
Kreusch, A., Pfaffinger, P.J., Stevens, C.F. & Choe, S. Nature 392, 945–948 (1998).
Bixby, K.A. et al. Nature Struct, Biol, 6, 38–43 (1999).
Stewart, R. in The Proton: Applications to organic chemistry 228–234 (Academic Press, Orlando, Florida; 1985).
Doublie, S. Methods Enzymol. 276, 523–530 (1997).
Otwinowski, Z. In Data collection and processing (ed. Sawyer, L., Isaacs, N. & Bailey, S.) 56–62 (, SERC Laboratory, Warrington, UK; 1993).
Jones, T.A., Zou, J.Y., Cowan, S.W. & Kjeldgaard . Acta Crystallogr A 47, 110–119 (1991).
Brunger, A.T. Nature 355, 472–474 (1992).
Rice, L.M. & Brunger, A.T. Proteins 19, 277–290 (1994).
Nicholls, A., Sharp, K.A. & Honig, B. Proteins 11, 281–296 (1991).
Laskowski, R.A., MacArthur, M.W., Moss, D.S. & Thornton, J.M. J. Appl. Cryst. 26, 283–291 (1993).
Acknowledgements
We are grateful to Z. Otwinowski for SeMet data collection and initial processing, H. Lee for help preparing crystallization and heavy-atom soak trials, R. Jain, S. Lockless, and M. Machius for insightful discussions, and J. Remington and R. Tsien for communication of data prior to publication. This work was partially supported by a grant from the Robert A. Welch Foundation to R.R., who is also a recipient of the Burroughs-Wellcome Fund New Investigator Award in the Basic Pharmacological Sciences, and is an Assistant Investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wall, M., Socolich, M. & Ranganathan, R. The structural basis for red fluorescence in the tetrameric GFP homolog DsRed. Nat Struct Mol Biol 7, 1133–1138 (2000). https://doi.org/10.1038/81992
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/81992
This article is cited by
-
Bioinformatic and literature assessment of toxicity and allergenicity of a CRISPR-Cas9 engineered gene drive to control Anopheles gambiae the mosquito vector of human malaria
Malaria Journal (2023)
-
A monomeric StayGold fluorescent protein
Nature Biotechnology (2023)
-
A mitotic chromatin phase transition prevents perforation by microtubules
Nature (2022)
-
Expression and Characterization of a Bright Far-red Fluorescent Protein from the Pink-Pigmented Tissues of Porites lobata
Marine Biotechnology (2020)
-
Positive functional synergy of structurally integrated artificial protein dimers assembled by Click chemistry
Communications Chemistry (2019)