Abstract
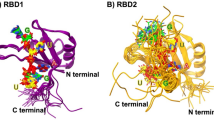
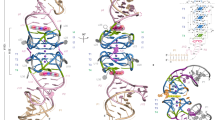
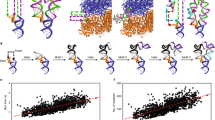
We have determined the crystal structures, at 2.8 Å resolution, of two different RNA aptamers, each bound to MS2 coat protein. One of the aptamers contains a non-Watson-Crick base pair, while the other is missing one of the unpaired adenines that make sequence-specific contacts in the wild-type complex. Despite these differences, the RNA aptamers bind in the same location on the protein as the wild-type translational operator. Comparison of these new structures with other MS2-RNA complexes allows us to refine further the definition of the minimal recognition elements and suggests a possible application of the MS2 system for routine structure determination of small nucleic acid motifs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Cusack, S. Nature Struct. Biology 2, 824–831 (1995).
Nagai, K. Curr. Op. Struc. Biol. 6, 53–61 (1996).
Uhlenbeck, O.C., Pardi, A. & Feigon, J. Cell 90, 833–840 (1997).
Convery, M.A. et al. Nature Struct. Biology 5, 133– 139 (1998).
Valegård, K., Murray, J.B., Stockley, P.G., Stonehouse, N.J. & Liljas, L. Nature 371, 623–626 (1994).
Valegård, K., et al. J. Mol. Biol. 270, 724– 738 (1997).
Witherell, G.W., Gott, J.M. & Uhlenbeck, O.C. Prog. Nuc. Acid Res. 40, 185 –220 (1991).
Stockley, P.G., et al. Nucleic Acids Res. 23, 2512– 2518 (1995).
Romaniuk, P.J., Lowary, P., Wu, H.N., Stormo, G. & Uhlenbeck, O.C. Biochemistry 26, 1563– 1568 (1987).
Patel, D.J., et al. J. Mol. Biol. 272, 645– 664 (1997).
Jaeger, J.A., Turner, D.H. & Zuker, M. Meth. Enzymol. 183, 281– 306 (1989).
Schneider, D., Tuerk, C. & Gold, L. J. Mol. Biol. 228, 862–869 (1992).
Scott, W.G., Finch, J.T. & Klug, A. Cell 81, 991–1002 (1995).
Cate, J.H., et al. Science 273, 1678– 1685 (1996).
Yoshizawa, S., Kawai, G., Watanabe, K., Miura, K. & Hirao I. Biochemistry 36, 4761–4767 (1997).
Leonard, G.A., et al. Structure 2, 483–494 (1994).
Wu, H.N. & Uhlenbeck, O.C. Biochemistry 26, 8221–8227 (1987).
Talbot, S.J., Goodman, S., Bates, S.R.E., Fishwick, C.W.G. & Stockley, P.G. Nucleic Acids Res. 18, 3521–3528 (1990).
Lago, H., Fonseca, S.A., Murray, J.B., Stonehouse, N.J. & Stockley, P.G. Nucleic Acids Res. 26, 1337–1344 (1998).
Nicholls, A., Sharp, K.A. & Honig, B. Proteins 11, 281– 296 (1991).
Golmohammadi, R., Valegård, K., Fridborg, K. & Liljas, L. J. Mol. Biol. 234, 620–639 (1993).
Borer, P.N., et al. Biochemistry 34, 6488– 6503 (1995).
Uhlenbeck, O.C. Nature Struct. Biology 5, 174–176 (1998).
Murray, J.B., Collier, A.K. & Arnold, J.R.P. Anal. Biochem. 218, 177– 184 (1994).
Esnouf, R.M. Journal of Molecular Graphics 15, 133– 138 (1997).
Bacon, D. & Anderson, W.F. Journal of Molecular Graphics 6, 219–220 ( 1988).
Merritt, E.A. & Murphy, M.E.P. Acta Crystallogr. D50, 869–873 (1994).
Acknowledgements
The authors would like to thank L. Liljas and members of his laboratory at Uppsala University, Sweden, for their assistance over a number of years with the crystallography of phage particles and for comments on the manuscript. We also thank J. Jäger for helpful discussions and S. Fonseca for help with protein purification. This work was supported by grants from the U.K. B.B.S.R.C. and M.R.C., the Wellcome Trust, the Leverhulme Trust and the Howard Hughes Medical Institute. N.J.S. is an M.R.C. fellow.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rowsell, S., Stonehouse, N., Convery, M. et al. Crystal structures of a series of RNA aptamers complexed to the same protein target . Nat Struct Mol Biol 5, 970–975 (1998). https://doi.org/10.1038/2946
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/2946
This article is cited by
-
In situ structures of the genome and genome-delivery apparatus in a single-stranded RNA virus
Nature (2017)
-
Asymmetric cryo-EM reconstruction of phage MS2 reveals genome structure in situ
Nature Communications (2016)
-
A novel method to produce armored double-stranded DNA by encapsulation of MS2 viral capsids
Applied Microbiology and Biotechnology (2015)
-
A quantitative framework for the forward design of synthetic miRNA circuits
Nature Methods (2014)
-
Analysis of stacking overlap in nucleic acid structures: algorithm and application
Journal of Computer-Aided Molecular Design (2014)