Abstract
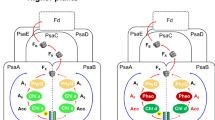
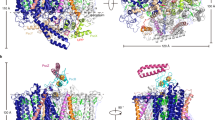
The 4 Å X-ray structure model of trimeric photosystem I of the cyanobacterium Synechococcus elongatus reveals 31 transmembrane, nine surface and three stromal α-helices per monomer, assigned to the 11 protein subunits: PsaA and PsaB are related by a pseudo two-fold axis normal to the membrane plane, along which the electron transfer pigments are arranged. 65 antenna chlorophyll a (Chl a) molecules separated by ≤16 Å form an oval, clustered net, continuous with the electron transfer chain through the second and third Chl a pairs of the electron transfer system. This suggests a dual role for these Chl a both in excitation energy and electron transfer. The architecture of the protein core indicates quinone and iron-sulphur type reaction centres to have a common ancestor.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Hill, R. & Bendall, F. Function of the two cytochrome components in chloroplasts: A working hypothesis. Nature 186, 136–137 (1960).
Kok, B. & Hoch, G. in Light and Life (eds W.D. McElroy & B. Glass) 397–405 (John Hopkins Press, 1961).
Duysens, L.N.M., Amesz, J. & Kamp, B.M. Two photochemical systems in photosynthesis. Nature 190, 510–511 (1961).
Witt, H.T., Müller, A. & Rumberg, B. Experimental evidence for the mechanism of photosynthesis. Nature 191, 194–195 (1961).
Yu, L., Zhao, J., Mühlenhoff, U., Bryant, D.A. & Golbeck, J.H. PsaE is required for in vivo cyclic electron flow around Photosystem I in the cyanobacerium Synechococcus sp. PCC7002. Plant Physiol. 103, 171–180 (1993).
Yu, L. et al in Res. Photosynth. Proclnt. Congr. Photosynth. 9th Ed. (Ed. N. Murata) 565–568 (Kluwer, Dordrecht, Netherlands, 1992).
Fork, D.C. & Herbert, S.K. Electron transport and photophosphorylation by Photosystem I in vivo in plants and cyanobacteria. Photosynth. Res. 36 149–168 (1993).
Deisenhofer, J., Epp, O., Miki, K., Huber, R. & Michel, H. Structure of the protein subunits in the photosynthetic reaction center of Rhodopseudomonas viridis at 3 Å resolution. Nature 318, 618–624 (1985).
Ermler, U., Fritsch, G., Buchannan, S.K. & Michel, H. Structure of the photosynthetic reaction centre from Rhodobacter sphaeroides at 2.65 Å resolution: cofactors and protein-cofactor interactions. Structure 2, 925–936 (1994)
Ducruix, A. & Reiss-Husson, F. Preliminary characterisation by X-ray diffraction of crystals of photochemical reaction centres from wild-type Rhodopseudomonas sphaeroides. J. Mot. Biol. 193, 419–421 (1987).
Alien, J.P., Feher, G., Yeates, T.O., Komiya, H. & Rees, D.C. Structure of the reaction center from Rhodobacter sphaeroides R-26: The protein subunits. Proc. Natl Acad. Sci. USA 84, 6162–6166 (1987).
Chang, C.H., el-Kabbani, O., Tiede, D., Morris, J. & Schiffer, M. Structure of the membrane-bound protein photosynthetic reaction center from Rhodobacter sphaeroides. Biochemistry 30, 5352–5360 (1991).
Golbeck, J.H. Photosystem I in cyanobacteria. in Molecular Biology of Cyanobacteria, (ed. D.A. Bryant) 179–220 (Kluwer, Dordrecht, 1994).
Chitnis, P.R., Xu, Q., Chitnis, V.P. & Nechushtai, R. Function and organisation of Photosystem I polypeptides. Photosynth. Res. 44, 23–40 (1995).
Mühlenhoff, U., Haehnel, W., Witt, H. & Herrmann, R.G. Genes encoding eleven subunits of Photosystem I from the thermophilic cyanobacterium Synechococcus sp. Gene 127, 71–78 (1993).
Hladík, J. & Sofrová, D. Does the trimeric form of the Photosystem 1 reaction center of cyanobacteria in vivo exist? Photosynthesis Research 29, 171–175 (1991).
Shubin, V.V., Tsuprun, V.L., Bezsmertnaya, I.N. & Karapetyan, N.B. Trimeric forms of the Photosystem I reaction center complex pre-exits in the membranes of cyanobacterium Spirulina platensis. FEBS Lett. 334, 79–82 (1993).
Tsiotis, G., Haase, W., Engel, A. & Michel, H. Isolation and structural characterisation of trimeric cyanobacterial Photosystem I complex with the help of recombinant antibody fragments. Eur. J. Biochem. 231, 823–830 (1995).
Rögner, M., Mühlenhoff, U., Boekema, E.J. & Witt, H.T. Mono-, di-, and trimeric PS I reaction center complexes isolated from the thermophilic cyanobacterium Synechococcus sp. Size, shape and activity. Biochim. Biophys. Acta 1015, 415–424 (1990).
Witt, I. et al. X-ray characterisation of single crystals of the reaction center I of water splitting photosynthesis. Ber. Bunsenges. Phys. Chem. 92, 1503–1506 (1988).
Witt, H.T., Krauß, N., Hinrichs, W., Witt, I., Fromme, P. & Saenger, W. Three-dimensional crystals of photosystem I from Synechococcus sp. and X-Ray structure analysis at 6 Å resolution., in Research in Photosynthesis., Vol 1 (ed. N. Murata) 521–528 (Kluwer, Dordrecht, 1992).
Krauß, N. et al. Three-dimensional structure of system I of photosynthesis at 6 Å resolution. Nature 361, 326–331 (1993).
Schubert, W.-D. et al. Present state of the crystal structure analysis of Photosystem I at 4.5 Å resolution. in Photosynthesis: from Light to Biosphere, 2 (ed. P. Mathis) 3–11 (Kluwer, Dordrecht, 1995).
Richardson, J.S. & Richardson, D.C. Interpretation of electron density maps. Meths Enzymol. 115, 189–206 (1985).
Adman, E.T., Sieker, L.C. & Jensen, L.H. The structure of Peptococcus aerogenes ferredoxin. Refinement at 2 Å resolution. J. Biol. Chem. 251, 3801–3806 (1976).
Falzone, C.J., Kao, Y.-H., Zhao, J., Bryant, D.A. & Lecomte, J.T.J. Three-dimensional solution structure of PsaE from the cyanobacterium Synechococcus sp. Strain PCC 7002, a Photosystem I protein that shows structural homology with SH3 Domains. Biochemistry 33, 6052–6062 (1994).
Xu, Q., Chitnis, V.P. & Chitnis, P.R. Structural organization of photosystem I polypeptides in Photosynthesis: from Light to Biosphere. 2 (ed. P. Mathis) 87–90 (Kluwer, Dordrecht, 1995).
Jansson, S. Cross-linking studies of PS I. Plant Physiol. in the press, (1996).
Jekow, P., Fromme, P., Witt, H.T. & Saenger, W. Photosystem I from Synechococcus elongatus: preparation and crystallization of monomers with varying subunit compositions. Biochim. Biophys. Acta 1229, 115–1120 (1995).
Boekema, E.J., Boonstra, A.F., Dekker, J.P. & Rögner, M. Electron microscopic structural analysis of Photosystem I, Photosystem II, and the cytochrome b6/f complex from green plants and cyanobacteria. J. Bioenerg. Biomem. 26, 17–29 (1994).
Böttcher, B., Gräber, P. & Boekema, E.J. The structure of Photosystem I from the thermophilic cyanobacterium Synechococcus sp. determined by electron microscopy of two-dimensional crystals. Biochim. Biophys. Acta 1100, 125–136 (1992).
Kruip, J., Boekema, E.J., Bald, D., Boonstra, A.F. & Rögner, M. Isolation and Structural Characterisation of Monomeric and Trimeric Photosystem I Complexes (P700.FA/FB and P700.FX) from the Cyanobacterium Synechocystis PCC 6803. J. Biol. Chem. 268, 23353–23360 (1993).
Rodday, S.M., Jun, S.-S. & Biggins, J. Interaction of the FAFB-containing subunit with the Photosystem 1 core heterodimer. Photosynth. Res. 36, 1–9 (1993).
Chitnis, V.P. et al. Targeted inactivation of the gene psaL encoding a subunit of Photosystem I of the cyanobacterium Synechocystis sp PCC 6803. J. Biol. Chem. 268, 11678–11684 (1993).
Okkels, J.S., Scheller, H.V., Svendsen, I. & Møller, B.L. Isolation and characterization of a cDNA clone encoding an 18-kDa hydrophobic Photosystem I subunit (PSI-L) from barley (Hordeum vulgare L.) J. Biol. Chem. 266, 6767–6773 (1991).
Xu, Q. et al. Mutational analysis of Photosystem I polypeptides in the cyanobacterium Synechocystis sp. PCC 6803. Targeted inactivation of psal reveals the function of Psal in the structural organization of PsaL. J. Biol. Chem. 270, 16243–16250 (1995).
Franzén, L.-G., Frank, G., Zuber, H. & Rochaix, J.-D. Isolation and characterization of cDNA clones encoding Photosystem I subunits with molecular masses 11.0, 10.0, and 8.4 kDa from Chlamydomonas reinhardtii. Mol. Gen. Genet. 219, 137–144 (1989).
Farah, J., Rappaport, F., Choquet, Y., Joliot, P. & Rochaix, J-D. Isolation of a psaF-deficient mutant of Chlamydomonas reinhardtii: efficient interaction of plastocyanin with the Photosystem I reaction center is mediated by the PsaF subunit. EMBO J. 14, 4976–4984 (1995).
Hippler, M., Ratajczak, R. & Haehnel, W. Identification of the plastocyanin-binding subunit of Photosystem I. FEBS Lett. 250, 280–284 (1989).
Kruip, J. et al. Localization of subunits in PS1, PS2 and in a PS2/Light-Harvesting-Supercomplex. in Photosynthesis: from Light to Biosphere., 3 (ed. P. Mathis) 405–408 (Kluwer, Dordrecht., 1995).
Golbeck, J.H., Parrett, K.G., Mehari, T., Jones, K.L. & Brand, J.J. Isolation of the intact Photosystem I reaction center core containing P700 and iron-sulfur center FX . FEBS Lett. 288, 268–272 (1988).
Rousseau, F. & Lagoutte, B. Evidence for the involvement of PSI-E subunit in the reduction of ferredoxin by Photosystem I. EMBO J. 12, 1755–1765 (1993).
Høj, P.B., Svendsen, I., Scheller, H.V., Møller, B.L. Identification of a chloroplast-encoded 9-kDa polypeptide as a 2[4Fe-4S] protein carrying centers A and B of Photosystem I. J. Biol. Chem. 262, 12676–12684 (1987).
Hayashida, N. et al. The gene for the 9 kDa polypeptide, a possible apoprotein for the iron-sulfur centers A and B of the Photosystem I complex, in tobacco chloroplast DNA. Curr. Genet. 12, 247–250 (1987).
Oh-oka, H. et al. The 8 kDa polypeptide in Photosystem I is a probable candidate of an iron-sulfur center protein coded by the chloroplast gene frxA. FEBS Lett. 218, 52–54 (1987).
Kamlowski, A., van der Est, A., Fromme, P. & Stehlik, D. Structural organization of the acceptors A1, FX, FA and FB in Photosystem I from EPR in solution and single crystals in Photosynthesis: from Light to Biosphere. 2 (ed. P. Mathis) 29–34 (Kluwer, Dordrecht, 1995).
Zhao Li, N., Warren, P.V., Golbeck, J.H. & Bryant, D.A. Site-Directed conversion of a cystein to aspartate leads to the assembly of a [3Fe-4S] cluster in PsaC of Photosystem I. The photoreduction of FA is independent of FB . Biochemistry 31, 5093–5099 (1992).
Käß, H., Bittersmann-Weidlich, E., Andreasson, L.-E., Boenigk, B. & Lubitz, W. ENDOR and ESEEM of the 15N labelled radical cations of chlorophyll a and the primary donor P700 in Photosystem I. Chem. Phys. 194, 419–432 (1995).
Evans, M.C.W., Bratt, P.J., Heathcote, P., Moënne-Loccoz, P., Fourier transform electron spin echo envelope modulation spectroscopy of the 14N primary donors, P700+ and P870+. in Photosynthesis: from Light to Biosphere. Vol. 2 (ed. P. Mathis), 183–186 (Kluwer, Dordrecht, 1995).
Arlt, T. et al. The accessory bacteriochlorophyll: a real electron carrier in primary photosynthesis. Proc. Natl. Acad Sci. USA 90, 11757–11761 (1993).
Brettel, K. New assignment for the 250 μs kinetics in Photosystem I: P-700* recombines with A1− (not FX−). Biochim. Biophys. Acta. 576, 246–249 (1989).
Flemming, C. Charakterisierung des Antennensystems von monomeren und trimeren Photosystem I Komplexen. Diploma Thesis, Technische Universität Berlin, 1996.
van Grondelle, R., Energy transfer and trapping in photosynthesis, Biochim. Biophys. Acta. 1187, 1–65, (1994).
Vermaas, W.F.J. Evolution of heliobacteria: implications for photosynthetic reaction center complexes. Photosynth. Res. 41, 285–294 (1994).
Fromme, P., Witt, H.T., Schubert, W.-D., Klukas, O., Saenger, W. & Krauß, N. Structure of Photosystem I at 4.5 Å resolution: A short review including evolutionary aspects. Biochim. Biophys. Acta 1275, 76–83 (1996).
Leslie, A.G.W. in Joint CCP4 and ESF-EACMB Newsletter on Protein Crystallography, No. 26, Daresbury Laboratory, Warrington, United Kingdom (1992).
Otwinowski, Z., Oscillation Data Reduction Protgramm, in Proceedings of the CCP4 Study Weekend: Data Collection and Processing (eds L. Sawyer, N. Isaacs, S. Bailey) 56–62 (SERC, Daresbury Laboratory, England, 1993).
CCP4, The Collaborative Computational Project, Number 4. The CCP4 Suite; Programms for Protein Crystallography. Acta Cryst. D50, 760–763 (1994).
Cowtan, K. Joint CCP4 and ESF-EACBM Newsletter on Protein Crystallography 31, 34–48 (1994).
Jones, T.A., Zou, J.Y., Cowan, S.W. & Kjeldgaard, M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Cryst. A47, 110–119 (1991).
Krabben, L. et al. Site-directed mutations of PsaB for the study of cofactor-protein and protein-protein interaction of Photosystem I. in Photosynthesis: from Light to Biosphere. Vol. 2 (ed. P. Mathis) 123–126 (Kluwer, Dordrecht, 1995).
Margulies, M.M. Sequence similarity between photosystems I and II. Identification of a Photosystem I reaction center transmembrane helix that is similar to transmembrane helix IV of the D2 subunit of Photosystem II and the M subunit of the non-sulfur purple and flexible green bacteria. Photosynth. Res. 29, 133–47 (1991).
Gavel, Y., Steppuhn, J., Herrmann, R.G. & von Heijne, G. The ‘positive-inside rule’ applies to thylakoid membrane proteins. FEBS Lett. 282, 41–16 (1991).
Vallon, O. & Bogograd, L. Topological study of PSI-A and PSI-B, the large subunits of the Photosystem-l reaction center. Eur. J. Biochem. 214, 907–915 (1993).
Kraulis, P.K. Molscript: a program to produce both detailed and schematic plots of protein structures. J. Appl. Crystallogr. 24, 946–950 (1991).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Krauß, N., Schubert, WD., Klukas, O. et al. Photosystem I at 4 Å resolution represents the first structural model of a joint photosynthetic reaction centre and core antenna system. Nat Struct Mol Biol 3, 965–973 (1996). https://doi.org/10.1038/nsb1196-965
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nsb1196-965
This article is cited by
-
Room temperature XFEL crystallography reveals asymmetry in the vicinity of the two phylloquinones in photosystem I
Scientific Reports (2021)
-
Membrane protein megahertz crystallography at the European XFEL
Nature Communications (2019)