Abstract
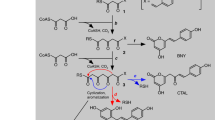
Chalcone isomerase (CHI) catalyzes the intramolecular cyclization of chalcone synthesized by chalcone synthase (CHS) into (2S)-naringenin, an essential compound in the biosynthesis of anthocyanin pigments, inducers of Rhizobium nodulation genes, and antimicrobial phytoalexins. The 1.85 Å resolution crystal structure of alfalfa CHI in complex with (2S)-naringenin reveals a novel open-faced β-sandwich fold. Currently, proteins with homologous primary sequences are found only in higher plants. The topology of the active site cleft defines the stereochemistry of the cyclization reaction. The structure and mutational analysis suggest a mechanism in which shape complementarity of the binding cleft locks the substrate into a constrained conformation that allows the reaction to proceed with a second-order rate constant approaching the diffusion controlled limit. This structure raises questions about the evolutionary history of this structurally unique plant enzyme.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Dooner, H.K., Robbins, T.P. & Jorgensen, R.A. Annu. Rev. Genet. 25, 173–199 (1991).
Long, S.R. Cell 56, 203–214 (1989).
Dixon, R.A. & Paiva, N.L. Plant Cell 7, 1085–1097 (1995).
Bohm, B.A. Introduction to Flavonoids (Harcourt, Singapore; 1998).
Setchell, K.D.R. & Cassidy, A. J. Nutr. 129, 758S–767S (1999).
Dixon, R.A. & Steele, C.J. Trends Plant Sci. 4, 394–400 (1999).
Davies, K.M., Bloor, S.J., Spiller, G.B. & Deroles, S.C. Plant J. 13, 259–266 (1998).
Jung, W. et al. Nature Biotech. 18, 208–212 (2000).
DellaPenna, D. Science 285, 375–379 (1999).
Ferrer, J.L., Jez, J.M., Bowman, M.E., Dixon, R.A. & Noel, J.P. Nature Struct. Biol. 6, 775–784 (1999).
van Tunen, A.J., Mur, L.A., Recourt, K., Gerats, A.G. & Mol, J.N. Plant Cell 3, 39–48 (1991).
Firmin, J.L., Wilson, K.E., Rossen, L. & Johnston, A.W.B. Nature 324, 90–92 (1986).
Bednar, R.A. & Hadcock, J.R. J. Biol. Chem. 263, 9582–9588 (1988).
Dixon, R.A., Blyden, E.R., Robbins, M.P., van Tunen, A.J. & Mol, J.N. Phytochemistry 27, 2801–2808 (1988).
McKhann, H.I. & Hirsch, A.M. Plant Mol. Biol. 24, 767–777 (1994).
Sander, C. & Schneider, R. Proteins 9, 56–68 (1991).
Altschul, S.F. et al. Nucleic Acids Res. 25, 3389–3402 (1997).
Bednar, R.A., Fried, W.B., Lock, Y.W. & Pramanik, B. J. Biol. Chem. 264, 14272–14276 (1989).
Hahlbrock, K., Wong, E., Schill, L. & Grisebach, H. Phytochemistry 9, 949–958 (1970).
Boland, M.J. & Wong, E. Bioorg. Chem. 8, 1–8 (1979).
Rathmell, W.G. & Bendall, D.S. Biochem. J. 127, 125–132 (1972).
Jencks, W.P. Catalysis in Chemistry and Enzymology (McGraw-Hill, New York; 1969).
Bruice, T.C. & Pandit, U.K. J. Am. Chem. Soc. 82, 5858–5865 (1960).
Page, M.I. & Jencks, W.P. Proc. Natl. Acad. Sci. USA 68, 1678–1683 (1971).
Winkel-Shirley, B. Physiol. Plant. 107, 142–149 (1999).
Hrazdina, G. & Wagner, G.J. Arch. Biochem. Biophys. 237, 88–100 (1985).
Hrazina, G., Zobel, A.M. & Hoch, H.C. Proc. Natl. Acad. Sci. USA 84, 8966–8970 (1987).
Burbulis, I.E. & Winkel-Shirley, B. Proc. Natl. Acad. Sci. USA 96, 12929–12934 (1999).
Graham, L.E., Cook, M.E. & Busse, J.S. Proc. Natl. Acad. Sci. USA 97, 4535–4540 (2000).
Jez, J.M., Ferrer, J.-L., Bowman, M.E., Dixon, R.A. & Noel, J.P. Biochemistry 39, 890–902 (2000).
Otwinowski, Z. & Minor, W. Methods Enzymol. 276, 307–326 (1997).
Collaborative Computational Project, Number 4. Acta Crystallogr. D 53, 240–255 (1994).
Terwilliger, T.C. & Berendzen, J. Acta Crystallogr. D 55, 849–861 (1999).
Otwinowski, Z. ML-PHARE in Daresbary Study Weekend Proceedings (CCP4, SERCDaresbary Laboratory, Warrington, UK; 1991).
de La Fortelle, E. & Bricogne, G. Methods Enzymol. 276, 472–494 (1997).
Abrahams, J.P. & Leslie, A.G.W. Acta Crystallogr. D 52, 30–42 (1996).
Jones, T.A., Zou, J.Y., Cowan, S.W. & Kjeldgaard, M. Acta Crystallogr. D 49, 148–157 (1993).
Brünger, A.T. et al. Acta Crystallogr. D 54, 905–921 (1998).
Laskowski, R.A., MacArthur, M.W., Moss, D.S. & Thornton, J.M. J. Appl. Crystallogr. 26, 283–291 (1993).
Kraulis, P.J. J. Appl. Crystallogr. 24, 946–950 (1991).
Nicholls, A., Sharp, K. & Honig, B. Proteins 11, 281–296 (1991).
Amundsen, S., et al. POV-Ray: persistence of vision ray-tracer. http://www.povray.org (1997).
Acknowledgements
We thank A. Hirsch (UCLA) for providing the CHI cDNA. The SSRL Biotechnology Program is supported by the NIH, National Center for Research Resources, Biomedical Technology Program, and the DOE, Office of Biological and Environmental Research. This work was supported by a grant from the National Science Foundation awarded to J.P.N. J.M.J. is a NIH Postdoctoral Research Fellow and also received support from the Hoffman Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jez, J., Bowman, M., Dixon, R. et al. Structure and mechanism of the evolutionarily unique plant enzyme chalcone isomerase. Nat Struct Mol Biol 7, 786–791 (2000). https://doi.org/10.1038/79025
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/79025
This article is cited by
-
Generation and characterisation of an Arabidopsis thaliana f3h/fls1/ans triple mutant that accumulates eriodictyol derivatives
BMC Plant Biology (2024)
-
Flavonoids are involved in salt tolerance through ROS scavenging in the halophyte Atriplex canescens
Plant Cell Reports (2024)
-
Discovery of isoflavone phytoalexins in wheat reveals an alternative route to isoflavonoid biosynthesis
Nature Communications (2023)
-
Molecular cloning and in silico analysis of chalcone isomerase from Polygonum minus
Molecular Biology Reports (2023)
-
Overexpression of sesame polyketide synthase A leads to abnormal pollen development in Arabidopsis
BMC Plant Biology (2022)