Abstract
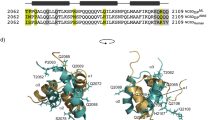
The tetrameric Mnt repressor is involved in the genetic switch between the lysogenic and lytic growth of Salmonella bacteriophage P22. The solution structure of its C-terminal tetramerization domain, which holds together the two dimeric DNA-binding domains, has been determined by NMR spectroscopy. This structure reveals an assembly of four α-helical subunits, consisting of a dimer of two antiparallel coiled coils with a unique right-handed twist. The superhelical winding is considerably stronger and the interhelical separation closer than those found in the well-known left-handed coiled coils in fibrous proteins and leucine zippers. An unusual asymmetry arises between the two monomers that comprise one right-handed coiled coil. A difference in the packing to the adjacent monomer of the other coiled coil occurs with an offset of two helical turns. The two asymmetric monomers within each coiled coil interconvert on a time scale of seconds. Both with respect to symmetry and handedness of helical packing, the C2 symmetric four-helix bundle of Mnt differs from other oligomerization domains that assemble DNA-binding modules, such as that in the tumor suppressor p53 and the E. coli lac repressor.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Crick, F.H.C. Acta. Crystallogr. 6, 685–689 (1953).
Crick, F.H.C. Acta. Crystallogr. 6, 689–697 (1953).
Lupas, A. Trends Biochem. Sci. 21, 375–382 (1996).
Kohn, W.D., Mant, C.T. & Hodges, R.S. J. Biol. Chem. 272, 2583– 2586 (1997).
Harbury, P.B., Plecs, J.J., Tidor, B., Alber, T. & Kim, P.S. Science 282, 1462– 1467 (1998).
Murzin, A.G., Brenner, S.E., Hubbard, T. & Chothia, C. J. Mol. Biol. 247, 536–540 (1995).
MacKenzie, K.R., Prestegard, J.H. & Engelman, D.M. Science 276, 131– 133 (1997).
Susskind, M.M. & Youderian, P. In Lambda II (eds Hendrix, R.W., Roberts, J.W., Stahl, F.W. & Weisberg, R.) 347– 366 (Cold Spring Harbor Press, Cold Spring Harbor, New York; 1983).
Waldburger, C.D. & Sauer, R.T. Biochemistry 34, 13109–13116 (1995).
Kabsch, W. & Sander, C. Biopolymers 22, 2577–2637 (1983).
O'Shea, E.K., Klemm, J.D., Kim, P.S. & Alber, T. Science 254, 539–544 (1991).
Gernert, K.M., Surles, M.C., Labean, T.H., Richardson, J.S. & Richardson, D.C. Protein Sci. 4, 2252–2260 (1995).
Knight, K.L. & Sauer, R.T. Biochemistry 27, 2088–2094 (1988).
Burgering, M.J. et al. Biochemistry 33, 15036– 15045 (1994).
Harbury, P.B., Zhang, T., Kim P.S. & Alber, T. Science 262, 1401–1407 (1993).
Lee, W. et al. Nature Struct. Biol. 1, 877– 890 (1994).
Clore, G.M. et al. Science 267, 1515– 1516 (1995).
Friedman, A.M., Fischmann, T.O. & Steitz, T.A. Science 268, 1721– 1727 (1995).
Harris, N.L. Presnell, S.R. & Cohen, F.E. J. Mol. Biol. 1356–1368 (1994).
Tan, S., Hunziker, Y., Sargent, D.F. & Richmond, T.J. Nature 381, 127–134 ( 1996).
Betz, S.F., Bryson, J.W. & DeGrado, W.F. Curr. Opin. Struct. Biol. 5, 457–463 (1995).
Nooren, I.M., George, A.V.E., Kaptein, R., Sauer, R.T. & Boelens, R. J. Biomol. NMR, in the press (1999).
Slijper, M., Kaptein, R. & Boelens, R. J. Magn. Reson. B 111, 199– 203 (1996).
Bax, A. & Davis, D.G. J. Magn. Reson. 63, 207–213 (1985).
Brünger, A.T. X-PLOR. A system for X-ray Crystallography and NMR (Yale University Press, New Haven, Connecticut; 1992).
Nilges, M. Proteins Struct. Funct. Genet. 17, 295– 309 (1993).
Nilges, M., Clore, G.M. & Gronenborn, A.M. FEBS Lett. 239, 129– 136 (1988).
Laskowski, R.A., MacArthur, M.W., Moss, D.S. & Thornton, J.M. J. Appl. Crystallogr. 26, 283–291 (1993).
Walther, D., Eisenhaber, F. & Argos, P. J. Mol. Biol. 255, 536– 553 (1996).
Koradi, R., Billeter, M. & Wüthrich, K. J. Mol. Graphics 14, 52– 55 (1996).
Milla, I.M., Brown, B.M. & Sauer, R.T., Protein Sci. 2, 2198– 2205 (1998).
Chothia, C., Levitt, M. & Richardson, D. J. Mol. Biol. 145, 215– 250 (1981).
Acknowledgements
This research was supported by the Council of Earth and Life Sciences of the Netherlands Organization for Scientific Research (ALW/NWO). We thank A.George and C. Waldburger for their help in protein expression and purification. D. Walther is gratefully acknowledged for his help in the analysis of helical parameters.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nooren, I., Kaptein, R., Sauer, R. et al. The tetramerization domain of the Mnt repressor consists of two right-handed coiled coils. Nat Struct Mol Biol 6, 755–759 (1999). https://doi.org/10.1038/11531
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/11531
This article is cited by
-
Drosophila Pif1A is essential for spermatogenesis and is the homolog of human CCDC157, a gene associated with idiopathic NOA
Cell Death & Disease (2019)
-
Genome Wide Analysis of WD40 Proteins in Saccharomyces cerevisiae and Their Orthologs in Candida albicans
The Protein Journal (2019)
-
Genome-wide association analysis identifies genetic variations in subjects with myalgic encephalomyelitis/chronic fatigue syndrome
Translational Psychiatry (2016)
-
RPRD1A and RPRD1B are human RNA polymerase II C-terminal domain scaffolds for Ser5 dephosphorylation
Nature Structural & Molecular Biology (2014)
-
Nature and mechanism of the in vivo oligomerization of nucleoid protein H-NS
The EMBO Journal (2005)