Abstract
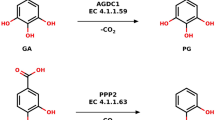
The structures of enzymes catalyzing the reactions in central metabolic pathways are generally well conserved as are their catalytic mechanisms. The two types of 3-dehydroquinate dehydratase (DHQase) are therefore most unusual since they are unrelated at the sequence level and they utilize completely different mechanisms to catalyze the same overall reaction. The type I enzymes catalyze a cis-dehydration of 3-dehydroquinate via a covalent imine intermediate, while the type II enzymes catalyze a trans-dehydration via an enolate intermediate. Here we report the three-dimensional structures of a representative member of each type of biosynthetic DHQase. Both enzymes function as part of the shikimate pathway, which is essential in microorganisms and plants for the biosynthesis of aromatic compounds including folate, ubiquinone and the aromatic amino acids. An explanation for the presence of two different enzymes catalyzing the same reaction is presented. The absence of the shikimate pathway in animals makes it an attractive target for antimicrobial agents. The availability of these two structures opens the way for the design of highly specific enzyme inhibitors with potential importance as selective therapeutic agents.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Chaudhuri, S., Duncan, K., Graham, L. D. & Coggins, J. R. Biochem. J. 275, 1–6 (1991).
Boys, C.W.G. et al. J. Mol. Biol. 227, 352–355 (1992).
Banner, D.W. et al. Nature 255, 609–614 (1975).
Sygusch, J., Beaudry, D. & Allaire, M. Proc. Natl. Acad. Sci. USA 84, 7846–7850 (1987).
Izard, T., Lawrence, M.C., Malby, R.L., Lilley, G.G. & Colman, P.M. Structure 2, 361–369 (1994).
Mirwaldt, C., Korndörfer, I. & Huber, R. J. Mol. Biol. 246, 227–239 (1995).
Burnett, R.M. et al. J. Biol. Chem. 249, 4383–4392. (1974).
Krell, T., Horsburgh, M.J., Cooper, A., Kelly, S.M. & Coggins, J.R. J. Biol. Chem. 271, 24492–24497 (1996).
Jia, J., Schörken, U., Lindqvist, Y., Sprenger, G.A. & Schneider, G. Protein Sci. 6, 119–124 (1997).
Reilly, A. et al. J. Biol. Chem. 269, 5523–5526 (1994).
Deka, R.K., Kleanthous, C. & Coggins, J.R. J. Biol. Chem. 267, 22237–22242 (1992).
Leech, A.P., James, R., Coggins, J.R. & Kleanthous, C. J. Biol. Chem. 270 25827–25836 (1995).
Bottomley, J.R., Hawkins, A.R. & Kleanthous, C. Biochem. J. 319, 269–278 (1996).
Harris, J.M., Gonzalez-Bello, C., Kleanthous, C., Hawkins, A.R, Coggins, J.R. & Abell,C. Biochem. J. 319, 333–336 (1996).
Price, N.C. et al. Biochem. J. 338, 195–202 (1999).
Hawkins, A.R., Lamb, H. K., Moore, J.D., Charles, I G. & Roberts, C.F. J. Gen. Microbiol. 139, 2891–2899 (1993).
Giles, N.H. et al. Microbiol. Rev. 49, 338–358 (1985).
Hawkins, A.R., Lamb, H.K., Smith, M., Keyte, J.W. & Roberts, C.F. Mol. Gen. Genet. 214, 224–231 (1988).
Giles, N.H., Partridge, C.W.H., Ahmed, S.I. & Case, M.E. Proc. Natl. Acad. Sci. USA 58, 1930–1937 (1967).
Hawkins, A.R., Giles, N.H. & Kinghorn, J.R. Biochem. Genet. 20, 271–286 (1982).
Euverink, G.J.W., Hessels, J.W., Vrijbloed, J.W., Coggins, J.R. & Dijkhuizen, L. J. General. Microbiol. 138, 2449–2457 (1992).
Philipp, W.J. et al. Proc. Natl. Acad. Sci. USA 93, 3132–3137 (1996).
Tomb, J.F. et al. Nature 388, 539–547 (1997).
Lamb, H.K. et al. Biochem.J. 313, 941–950 (1996).
Haslam, E. Shikimic acid: metabolism and metabolites (J. Wiley & Sons, Chichester, England; 1993).
Mousdale, D.M. & Coggins, J.R. (1991) In Target sites for herbicide action (ed. Kirkwood, R. C.) 29–56 (Plenum Press, New York; 1991).
Balasubramanian, S.G.M., Davies, G.M., Coggins, J.R. & Abell, C. J. Am. Chem. Soc. 113, 8945–8946 (1991).
Davies, G.M. et al. Antimicrob. Agents Chemother. 38, 403–406 (1994).
Tacket, C.O. et al. Vaccine 10, 443–446 (1992).
Karnell, A. et al. Vaccine 11, 830–836 (1993).
Otwinowski, Z. & Minor, W. Methods. Enzymol. 276, 307–326 (1997).
Collaborative Computational Project, Number 4. Acta Crystallogr. D 50, 760–763 (1994).
Brünger, A. T. X-PLOR Manual version 3.1: A system for crystallography and NMR. (Yale University Press, New Haven, Connecticut; 1992).
Otwinowski, Z. In Isomorphous replacement and anomalous scattering. (eds. Wolf, W. Evans, P.R. & Leslie, A.G.W.) 80–85 (SERC Daresbury Laboratory, Warrington, UK; 1991).
Cowtan, K. Joint CCP4 and ESF-EACBM Newsletter on protein crystallography. 31, 24–28 (1994).
Jones, T.A., Zou, J.-Y., Cowan, S. W. & Kjelgaard, M. Acta Crystallogr. A 47, 110–119 (1991).
Gourley, D.G. et al. J. Mol. Biol. 241, 488–491 (1994).
Murshudov, G.N., Dodson, E.J. & Vagin, A.A. In Macromolecular refinement (eds Dodson, E., Moore, M. & Bailey, S.) 93–104 (SERC Daresbury Laboratory, Warrington, UK; 1996).
Lamzin, V.S. & Wilson, K.S. Acta Crystallogr. D 49, 129–147 (1993).
Kraulis, P.J. J. Appl. Crystallogr. 24, 946–950 (1991).
Acknowledgements
We thank S. Bury, B. Boys, A. Lapthorn, J. Milner-White, G. Murshudov and L.A.A. Meira for help at various stages of the work and the staff at the SRS Daresbury, Warrington, UK and the EMBL Outstation, DESY, Hamburg for providing data collection facilities. This work was supported by the Biotechnology and Biological Science Research Council.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Gourley, D., Shrive, A., Polikarpov, I. et al. The two types of 3-dehydroquinase have distinct structures but catalyze the same overall reaction. Nat Struct Mol Biol 6, 521–525 (1999). https://doi.org/10.1038/9287
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/9287
This article is cited by
-
Revisiting the shikimate pathway and highlighting their enzyme inhibitors
Phytochemistry Reviews (2023)
-
Architecture and functional dynamics of the pentafunctional AROM complex
Nature Chemical Biology (2020)
-
Unraveling the kinetic diversity of microbial 3-dehydroquinate dehydratases of shikimate pathway
AMB Express (2015)
-
Crystal structure of a type II dehydroquinate dehydratase-like protein from Bifidobacterium longum
Journal of Structural and Functional Genomics (2013)
-
New insights into the mechanism of the Schiff base formation catalyzed by type I dehydroquinate dehydratase from S. enterica
Theoretical Chemistry Accounts (2012)