Abstract
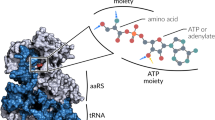
Glutamyl-tRNA synthetases (GluRSs) are divided into two distinct types, with regard to the presence or absence of glutaminyl-tRNA synthetase (GlnRS) in the genetic translation systems. In the original 19-synthetase systems lacking GlnRS, the 'non-discriminating' GluRS glutamylates both tRNAGlu and tRNAGln. In contrast, in the evolved 20-synthetase systems with GlnRS, the 'discriminating' GluRS aminoacylates only tRNAGlu. Here we report the 2.4 Å resolution crystal structure of a 'discriminating' GluRS·tRNAGlu complex from Thermus thermophilus. The GluRS recognizes the tRNAGlu anticodon bases via two α-helical domains, maintaining the base stacking. We show that the discrimination between the Glu and Gln anticodons (34YUC36 and 34YUG36, respectively) is achieved by a single arginine residue (Arg 358). The mutation of Arg 358 to Gln resulted in a GluRS that does not discriminate between the Glu and Gln anticodons. This change mimics the reverse course of GluRS evolution from anticodon 'non-dicsriminating' to 'discriminating'.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Accession codes
References
Lapointe, J., Duplain, L. & Proulx, M. J. Bacteriol. 165, 88–93 (1986).
Schön, A., Kannangara, G., Gough, S. & Söll, D. Nature 331, 187–190 (1988).
Rogers, K.C. & Söll, D. J. Mol. Evol. 40, 476–481 (1995).
Gagnon, Y., Lacoste, L., Champagne, N. & Lapointe, J. J. Biol.Chem. 271, 14856–14863 (1996).
Lamour, V. et al. Proc. Natl. Acad. Sci. U. S. A. 91, 8670–8674 (1994).
Siatecka, M., Rozek, M., Barciszewski, J. & Mirande, M. Eur. J. Biochem. 256, 80–87 (1998).
Curnow, A.W., Tumbula, D.L., Pelaschier, J.T., Min, B. & Söll, D. Proc. Natl. Acad. Sci. U. S. A. 95, 12838–12843 (1998).
Becker, H.D. & Kern, D. Proc. Natl. Acad. Sci. U. S. A. 95, 12832–12837 (1998).
Handy, J. & Doolittle, R.F. J. Mol. Evol. 49, 709–715 (1999).
Hara-Yokoyama, M., Yokoyama, S. & Miyazawa, T. J. Biochem. 96, 1599–1607 (1984).
Nureki, O. et al. Science 267, 1958–1965 (1995).
Robertus, J.D. et al. Nature 250, 546–551 (1974).
Kim, S.H. et al. Science 185, 435–440 (1974).
Rould, M.A., Perona, J.J., Söll, D. & Steitz, T.A. Science 246, 1135–1142 (1989).
Rould, M.A., Perona, J.J. & Steitz, T.A. Nature 352, 213–218 (1991).
Madore, E., et al. Eur. J. Biochem. 266, 1128–1135 (1999).
Brown, J.R. & Doolittle, W.F. J. Mol. Evol. 49, 485–495 (1999).
Tomb, J.F. et al. Nature 388, 539–547 (1997).
Curnow, A.W., Ibba, M. & Söll, D. Nature 382, 589–590 (1996).
Cavarelli, J., Rees, B., Ruff, M., Thierry, J.-C. & Moras, D. Nature 362, 181–184 (1993).
Schmitt, E. et al. EMBO J. 17, 5227–5237 (1998).
Auld, D.S. & Schimmel, P. Science 267, 1994–1996 (1995).
Auld, D.S. & Schimmel, P. EMBO J. 15, 1142–1148 (1996).
Sekine, S. et al. J. Mol. Biol. 256, 685–700 (1996).
Sekine, S., Nureki, O., Tateno, M. & Yokoyama, S. Eur. J. Biochem. 261, 354–360 (1999).
Otwinowski, Z. & Minor, W. In Methods Enzymol., Vol. 276. (eds. Carter, C.W.J. & Sweet, R.M.) 307–325 (Academic Press, London; 1997).
CCP4 Acta Cryst. D50, 760–763 (1994).
Jones, T.A., Zou, J.-Y., Cowan, S.W. & Kjeldgaard, M. Acta Cryst. A47, 110–119 (1991).
Brünger, A.T. X-PLOR: a system for X-ray crystallography and NMR. (Yale Univ. Press, New Haven; 1992).
Laskowski, R.A., MacArthur, M.W., Moss, D.S. & Thornton, J.M. J. Appl. Cryst. 26, 283–291 (1993).
Sugiura, I. et al. Structure Fold. Des. 8, 197–208 (2000).
Kraulis, P.J. J. Appl. Cryst. 24, 946–950 (1991).
Merritt, E.A. & Murphy, M.E.P. Acta Cryst. D50, 869–873 (1994).
Acknowledgements
S.Y. is the recipient of Grants-in-Aid for Science Research on Priority Areas from the Ministry of Education, Science, Sports and Culture of Japan; S.S. was supported by grants from the JSPS Research Fellowships for Young Scientists and from the RIKEN Special Postdoctoral Researchers Program.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Sekine, Si., Nureki, O., Shimada, A. et al. Structural basis for anticodon recognition by discriminating glutamyl-tRNA synthetase. Nat Struct Mol Biol 8, 203–206 (2001). https://doi.org/10.1038/84927
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/84927
This article is cited by
-
High-resolution crystal structure of human asparagine synthetase enables analysis of inhibitor binding and selectivity
Communications Biology (2019)
-
Structural basis for tRNA modification by Elp3 from Dehalococcoides mccartyi
Nature Structural & Molecular Biology (2016)
-
Evolutionary insights about bacterial GlxRS from whole genome analyses: is GluRS2 a chimera?
BMC Evolutionary Biology (2014)
-
Microbial production and applications of 5-aminolevulinic acid
Applied Microbiology and Biotechnology (2014)
-
An Investigation into Membrane Bound Redox Carriers Involved in Energy Transduction Mechanism in Brevibacterium linens DSM 20158 with Unsequenced Genome
The Journal of Membrane Biology (2014)