Abstract
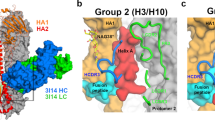
The structure of the hemagglutinin (HA) of a mutant influenza virus that escapes neutralization by a monoclonal antibody shows that the mutation causes changes in HA structure which avoid an energetically less favorable conformation. However, the structure of the mutant HA·Fab complex indicates that the antibody binds selectively to mutant HA in a wild type-like distorted conformation. The association of an antibody with a less favored HA conformation represents an alternative to previously described mechanisms of escape from neutralization by antibodies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Skehel, J. J. et al. Cold Spring Harbor Symposia on Quantitative Biology 60, 573–580 (1995)
Wiley, D. C. & Skehel, J. J. Annu. Rev. Biochem. 56, 365–394 (1987)
Wilson, I. A. & Cox, N. J .Ann. Rev. Immunol. 8, 737–771 (1990)
Bizebard, T. et al. Nature 376, 92–94 (1995)
Knossow, M., Daniels, R. S., Douglas, A. R., Skehel, J. J. & Wiley, D. C. Nature, 311, 678–680 (1984)
Bhat, T. N., Bentley, G. A., Fischmann, T. O., Boulot, G. & Poljak, R. J. Nature 347, 483–485 (1990).
Clackson, T. & Wells, J. A. Science 267, 383–386 (1995)
Nuss, J. M., Whitaker, P. B. & Air, G. M. Proteins 15, 121–132 (1993)
Colman, P. M. Prot. Sci. 3, 1687–1696 (1994)
Lea, S. et al. Structure 3, 571–580 (1995).
Cherfils, J., Bizebard, T., Knossow, M. & Janin, J. Proteins 18, 8–18 (1994)
Creighton, T. E. Prot Creighton, eins: structures and molecular properties, 2nd edition. (W.H. Freeman and Company, New York; 1993)
Parry, N. et al. Nature 347, 569–572 (1990)
Page, G. S. et al. J. Virol. 62, 1781–1794 (1988)
Tronrud, D. E, Holden, H. M. & Matthews, B.W. Science 235, 571–574 (1987)
Fersht, A. R. Biochemistry 27, 1577–1580 (1988)
Fields, B. A. et al. Biochemistry 35, 15494–15503 (1996)
Bizebard, T. et al. Acta Crystallographica D50, 768–777 (1994).
Temoltzin-Palacios, F. & Thomas, D. B. J. Exp. Med. 179, 1719–1724 (1994).
Friguet, B., Djavadi-Ohaniance, L & Goldberg, M. E. In Protein structure: a practical approach, (ed. Creighton, I.E.) 287–309 (IRL Press, Oxford; 1989).
Otwinowsky, Z. & Minor, W. Meth. Enz. 276, 307–325 (1997)
Jones, T. A.,, Zhou, J.-Y., Cowan, S. W. & Kjeldgaard, M. Acta. Crystallogr. A47, 110–119 (1991)
Jones, T. A. Meth. Enz. 115, 157–171 (1985)
Brünger, A. T. X–PLOR (VERSION 3.1) manual. (Yale University, New Haven, Connecticut, USA; 1992).
Laskowski, R. A., McArthur, M. W., Moss, D. S. & Thornton, J. M. J. Appl. Crystallogr. 26, 283–291 (1993)
Bernstein, F. C. et al. J. Mol. Biol. 112, 535–542 (1977)
Kabsch, W. Acta. Crystallogr. A32, 922–923 (1976)
Shrake, A. & Rupley, J. A. J. Mol. Biol. 79, 351–371 (1973).
Navaza, J. Acta. Crystallogr. A 50, 157–163 (1994)
Blundell, T. L. & Johnson, L. N. Protein crystallography. (Academic Press, London; 1976)
Kraulis, P. J. Appl. Crystallogr. 24, 924–950 (1991)
Brünger, A. T. Nature 355, 472–475 (1992)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fleury, D., Wharton, S., Skehel, J. et al. Antigen distortion allows influenza virus to escape neutralization. Nat Struct Mol Biol 5, 119–123 (1998). https://doi.org/10.1038/nsb0298-119
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsb0298-119
This article is cited by
-
Identification of a cross-neutralizing antibody that targets the receptor binding site of H1N1 and H5N1 influenza viruses
Nature Communications (2022)
-
Potential neutralizing antibodies discovered for novel corona virus using machine learning
Scientific Reports (2021)
-
Cross-neutralization of influenza A viruses mediated by a single antibody loop
Nature (2012)
-
"Boom" and "Bust" cycles in virus growth suggest multiple selective forces in influenza a evolution
Virology Journal (2011)
-
In silico characterization of the functional and structural modules of the hemagglutinin protein from the swine-origin influenza virus A (H1N1)-2009
Science China Life Sciences (2010)