Abstract
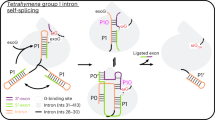
Most large RNAs achieve their active, native structures only as complexes with one or more cofactor proteins. By varying the Mg2+ concentration, the catalytic core of the bI5 group I intron RNA can be manipulated into one of three states, expanded, collapsed or native, or into balanced equilibria between these states. Under near-physiological conditions, the bI5 RNA folds rapidly to a collapsed but non-native state. Hydroxyl radical footprinting demonstrates that assembly with the CBP2 protein cofactor chases the RNA from the collapsed state to the native state. In contrast, CBP2 also binds to the RNA in the expanded state to form many non-native interactions. This structural picture is reinforced by functional splicing experiments showing that RNA in an expanded state forms a non-productive, kinetically trapped complex with CBP2. Thus, rapid folding to the collapsed state functions to self-chaperone bI5 RNA folding by preventing premature interaction with its protein cofactor. This productive, self-chaperoning role for RNA collapsed states may be especially important to avert misassembly of large multi-component RNA–protein machines in the cell.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lewis, J.D. & Tollervey, D. Science 288, 1385–1389 (2000).
Gluick, T.C. & Draper, D.E. J. Mol. Biol. 241, 246–262 (1994).
Wu, M. & Tinoco, I. Proc. Natl. Acad. Sci USA 95, 11555–11560 (1998).
Chamberlin, S.I. & Weeks, K.M. J. Am. Chem. Soc. 122, 216–224 (2000).
Sclavi, B., Sullivan, M., Chance, M.R., Brenowitz, M. & Woodson, S.A. Science 279, 1940–1943 (1998).
Powers, T., Daubresse, G. & Noller, H.F. J. Mol. Biol. 232, 362–374 (1993).
Weeks, K.M. Curr. Opin. Struct. Biol. 7, 336–342 (1997).
Held, W.A., Ballou, B., Mizushima, S. & Nomura, M. J. Biol. Chem. 249, 3103–3111 (1974).
Buchmueller, K.L., Webb, A.E., Richardson, D.A. & Weeks, K.M. Nature Struct. Biol. 7, 362–366 (2000).
Russell, R., Millett, I.S., Doniach, S. & Herschlag, D. Nature Struct. Biol. 7, 367–370 (2000).
Fang, X. et al. Biochemistry 39, 11107–11113 (2000).
Sosnick, T.R., Mayne, L., Hiller, R. & Englander, S.W. Nature Struct. Biol. 1, 149–156 (1994).
Dill, K.A. & Chan, H.S. Nature Struct. Biol. 4, 10–19 (1997).
Dobson, C.M. & Karplus, M. Curr. Opin. Struct. Biol. 9, 92–101 (1999).
Pan, J., Thirumalai, D. & Woodson, S.A. J. Mol. Biol. 273, 7–13 (1997).
Treiber, D.K. & Williamson, J.R. Curr. Opin. Struct. Biol. 9, 339–345 (1999).
Fang, X., Pan, T. & Sosnick, T.R. Nature Struct. Biol. 6, 1091–1095 (1999).
Rook, M.S., Treiber, D.K. & Williamson, J.R. J. Mol. Biol. 281, 609–620 (1998).
Hill, J., McGraw, P. & Tzagoloff, A. J. Biol. Chem. 260, 3235–3238 (1985).
Weeks, K.M. & Cech, T.R. Cell 82, 221–230 (1995).
Jaeger, L., Westhof, E. & Michel, F. J. Mol. Biol. 221, 1153–1164 (1991).
Weeks, K.M. & Cech, T.R. Biochemistry 34, 7728–7738 (1995).
Fersht, A. Enzyme Structure and Mechanism (Freeman and Co., New York; 1985).
Weeks, K.M. & Cech, T.R. Science 271, 345–348 (1996).
Costa, M. & Michel, F. EMBO J. 14, 1276–1285 (1995).
Michel, F. & Westhof, E. J. Mol. Biol. 216, 585–610 (1990).
Lehnert, V., Jaeger, L., Michel, F. & Westhof, E. Chem. Biol. 3, 993–1009 (1996).
Golden, B.L., Gooding, A.R., Podell, E.R. & Cech, T.R. Science 282, 259–264 (1998).
Gish, G. & Eckstein, F. Science 240, 1520–1522 (1988).
Acknowledgements
This work was supported by grants from the Searle Scholars Program of the Chicago Community Trust and by the NIH to K.M.W. We thank E. Westhof and C. Massire for modeling the bI5 group I intron RNA and P. Bevilacqua, M. Redinbo and K. Buchmueller for comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Webb, A., Weeks, K. A collapsed state functions to self-chaperone RNA folding into a native ribonucleoprotein complex. Nat Struct Mol Biol 8, 135–140 (2001). https://doi.org/10.1038/84124
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/84124
This article is cited by
-
Time-resolved RNA SHAPE chemistry: quantitative RNA structure analysis in one-second snapshots and at single-nucleotide resolution
Nature Protocols (2009)
-
Strategies for RNA folding and assembly
Nature Reviews Molecular Cell Biology (2004)