Abstract
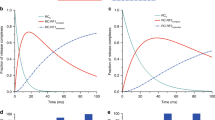
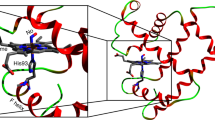
Cytochrome c folding was initiated using a new solution mixer that provides a time window which covers over 90% of the burst phase unresolved by conventional stop-flow measurements. Folding was followed by resonance Raman scattering. Kinetic analysis of the high frequency Raman data indicates that a nascent phase occurs within the mixing dead time of 100 μs. A significant fraction of the protein was found to be trapped in a misfolded bis-histidine form during the nascent phase at pH 4.5, thereby preventing the protein from folding rapidly and homogeneously. The nascent phase was followed by a haem-ligand exchange phase that populates the native histidine-methionine coordinated form through a thermodynamically controlled equilibrium.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Zwanzig, R., Szabo, A. & Bagchi, B. Levinthal's paradox. Proc. Natl. Acad. Sci. USA 89, 20–22 (1992).
Evans, P.A. & Radford, S.E. Probing the structure of folding intermediates. Curr. Opin. Struct. Biol. 4, 100–106 (1994).
Matthews, C.R. Pathways of Protein Folding, Annu. Rev. Biochem. 62, 653–683 (1993).
Sosnick, T.R., Mayene, L. & Englander, S.W. Molecular collapse: the rate-limiting step in two-state cytochrome c folding. Proteins Struct. Func. Genet. 24, 413–426 (1996).
Phillips, C.M., Mizutani, Y. & Hochstrasser, R.M. Ultrafast thermally induced unfolding of Rnase A. Proc. Nat. Acad. Sci. USA 92, 7292–7296 (1995).
Nolting, B., Golbik, P. & Fersht, A.R. Submillisecond events in protein folding. Proc. Nat. Acad. Sci. USA 92, 10668–10672 (1995).
Williams, S. et al. Fast events in protein folding: helix melting and formation in a small peptide. Biochemistry 35, 691–697 (1995).
Ballew, R.M., Sabelko, J. & Gruebele, M. Direct observation of fast protein folding: the initial collapse of apomyoglobin. Proc. Nat. Acad. Sci. USA 93, 5759–5764 (1996).
Jones, C.M. et al. Fast events in protein folding initiated by nanosecond laser photolysis. Proc. Nat. Acad. Sci. USA 90, 11860–11864 (1993).
Pascher, T., Chesick, J.P., Winkler, J.R. & Gray, H.B. Protein folding triggered by electron transfer. Science 271, 1558–1560 (1996).
Regenfuss, P., Clegg, R.M., Fulwyler, M.J., Barrantes, F.J. & Jovin, T.M. Mixing liquids in microseconds. Rev. Sci. Instrum. 56, 283–290 (1985).
Takahashi, S., Ching, Y.-c., Wang, J. & Rousseau, D.L. Microsecond generation of oxygen-bound cytochrome c oxidase by rapid solution mixing. J. Biol. Chem. 270, 8405–8407 (1995).
Bai, Y., Sosnick, T.R., Mayne, L. & Englander, S.W. Protein folding intermediates: native state hydrogen exchange. Science 269, 192–197 (1995).
Elove, G.A., Bhuyan, A.K. & Roder, H. Kinetic mechanism of cytochrome c folding: involvement of the heme and its ligands, Biochemistry 33, 6925–6935 (1994).
Sosnick, T.R., Mayene, L., Hiller, R. & Englander, S.W. The barriers in protein folding. Nature Struct. Biol. 1, 149–156 (1994).
Lehrer, S.S. Solute perturbation of protein fluorescence: the quenching of the tryptophyl fluorescence of model compounds and lysozyme by iodide ion. Biochemistry 10, 3254–3263 (1971).
Peterman, B.F. Measurement of the dead time of a fluorescence stopped-flow instrument. Anal. Biochem. 93, 442–444 (1979).
Tsong, T.Y. An acid induced conformational transition of denatured cytochrome c in urea and guanidine hydrochloride solutions. Biochemistry 14, 1542–1547 (1975).
Hu, S., Morris, I.K., Singh, J.P., Smith, K.M. & Spiro, T.G. Complete assignment of cytochrome c resonance Raman spectra via enzymatic reconstitution with isotopically labeled hemes. J. Am. Chem. Soc. 115, 12446–12458 (1993).
Jordan, T., Eads, J.C. & Spiro, T.G. Secondary and tertiary structure of the A-state of cytochrome c from resonance Raman spectroscopy. Protein Sci. 4, 716–728 (1995).
Chan, C.-K. et al. Submillisecond protein folding kinetics studied by ultrarapid mixing. Proc. Ntal. Acad. Sci. USA, in the press.
Peisach, J., Mims, W.B. & Davis, J.L. Water coordination by heme iron in metmyoglobin. J. Biol. Chem. 259, 2704–2706 (1984).
Ikeda-Saito, M. et al. Coordination structure of the ferric heme iron in engineered distal histidine myoglobin mutants. J. Biol. Chem. 267, 22843–22852 (1992).
Morikis, D., Champion, P.M., Springer, B.A., Egeberg, K.D. & Sliger, S.G. Raman studies of iron spin and axial coordination in distal pocket mutants of ferric myoglobin. J. Biol. Chem. 265, 12143–12145 (1990).
Quillin, M.L., Arduini, R.M., Olson, J.S. & Phillips Jr., G.N. High resolution crystal structures of distal histidine mutants of sperm whale myoglobin. J. Mol. Biol. 234, 140–155 (1993).
Brancaccio, A. et al. Structural factors governing azide and cyanide binding to mammalian metmyoglobins. J. Biol. Chem. 269, 13843–13853 (1994).
Wang, J.-S. & Van Wart, H.E. Resonance Raman characterization of the heme c group in N-acetyl-microperoxidase-8: a thermal intermediate spin-high spin state mixture. J. Phys. Chem. 93, 7925–7931 (1989).
Yeh, S.-R., Takahashi, S., Fan, B. & Rousseau, D.L. Ligand exchange during cytochrome c folding. Nature Struct. Biol. 4, 51–56 (1997).
Gottfried, D.S. et al. Evidence for damped hemoglobin dynamics in a room temperature trehalose glass. J. Phys. Chem. 100, 12034–12042 (1996).
Das, T.K. & Mazumdar, S. Conformational substates of apoprotein of horseradish peroxidase in aqueous solution: a fluorescence dynamics study. J. Phys. Chem. 99, 13283–13290 (1995).
Bushnell, G.W., Louie, G.V. & Brayer, G.D. High resolution three dimensional structure of horse heart cytochrome c. J. Mol. Biol. 214, 585–595 (1990).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Takahashi, S., Yeh, SR., Das, T. et al. Folding of cytochrome c initiated by submillisecond mixing. Nat Struct Mol Biol 4, 44–50 (1997). https://doi.org/10.1038/nsb0197-44
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nsb0197-44