Abstract
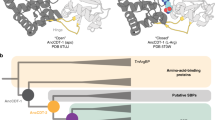
ThiS is a sulfur carrier protein that plays a central role in thiamin biosynthesis in Escherichia coli. Here we report the solution NMR structure of ThiS, the first for this class of sulfur carrier proteins. Although ThiS shares only 14% sequence identity with ubiquitin, it possesses the ubiquitin fold. This structural homology, combined with established functional similarities involving sulfur chemistry, demonstrates that the eukaryotic ubiquitin and the prokaryotic ThiS evolved from a common ancestor. This illustrates how structure determination is essential in establishing evolutionary links between proteins in which structure and function have been conserved through eons of evolution despite loss of sequence identity. The ThiS structure reveals both hydrophobic and electrostatic surface features that are likely determinants for interactions with binding partners. Comparison with surface features of ubiquitin and ubiquitin homologs SUMO-1, RUB-1 and NEDD8 suggest how Nature has utilized this single fold to incorporate similar chemistry into a broad array of highly specific biological processes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Hershko, A. & Ciechanover, A. Annu. Rev. Biochem. 67, 425–479 ( 1998).
Chen, Z.J., Parent, L. & Maniatis, T. Cell 84, 853– 862 (1996).
Haas, A.L. & Siepmann, T.J. Faseb J. 11, 1257–1268 (1997).
Bochtler, M., Ditzel, L., Groll, M., Hartmann, C. & Huber, R. Annu. Rev. Biophys. Biomol. Struct. 28, 295–317 (1999).
Furukawa, K., Mizushima, N., Noda, T. & Ohsumi, Y. J. Biol. Chem. 275, 7462–7465 ( 2000).
Jentsch, S. & Pyrowolakis, G. Trends Cell Biol. 10, 335–342 ( 2000).
Hochstrasser, M. Nature Cell Biol. 2, E153–E157 (2000).
Hochstrasser, M. Science 289, 563–564 ( 2000).
Begley, T.P., Xi, J., Kinsland, C., Taylor, S. & McLafferty, F. Curr. Opin. Chem. Biol. 3, 623–629 (1999).
Taylor, S.V. et al. J. Biol. Chem. 273, 16555– 16560 (1998).
Vijay-Kumar, S., Bugg, C.E. & Cook, W.J. J. Mol. Biol. 194, 531– 544 (1987).
Holm, L. & Sander, C. J. Mol. Biol. 233, 123–138 (1993).
Kraulis, P.J. Science 254, 581–582 ( 1991).
Tsukihara, T. et al. J. Mol. Biol. 216, 399– 410 (1990).
Russell, R.B., Saqi, M.A., Sayle, R.A., Bates, P.A. & Sternberg, M.J. J. Mol. Biol. 269, 423–439 (1997).
Rajagopalan, K.V. In Escherichia coli and Salmonella cellular and molecular biology, Vol. 1 (ed., Neidhardt, F.C.) 674– 686 (ASM Press, Washington, DC; 1996).
Unkles, S.E., Heck, I.S., Appleyard, M.V. & Kinghorn, J.R. J. Biol. Chem. 274, 19286–19293 (1999).
Pitterle, D.M. & Rajagopalan, K.V. J Biol Chem 268, 13499–13505 ( 1993).
Appleyard, M.V. et al. J. Biol. Chem. 273, 14869– 14876 (1998).
Lo Conte, L. et al. Nucleic Acids Res. 28, 257– 259 (2000).
Begley, T.P. et al. Arch. Microbiol. 171, 293– 300 (1999).
Miura, T., Klaus, W., Gsell, B., Miyamoto, C. & Senn, H. J. Mol. Biol. 290, 213–228 (1999).
Liu, Q. et al. J. Biol. Chem. 274, 16979– 16987 (1999).
Bayer, P. et al. J. Mol. Biol. 280, 275– 286 (1998).
Burch, T.J. & Haas, A.L. Biochemistry 33, 7300–7308 (1994).
Kinsland, C., Taylor, S.V., Kelleher, N.L., McLafferty, F.W. & Begley, T.P. Protein Sci. 7, 1839–1842 ( 1998).
Cornilescu, G., Delaglio, F. & Bax, A. J. Biomol. NMR 13, 289–302 (1999).
Cavanagh, J., Fairbrother, W.J., Palmer, A.G. & Skelton, N.J. Protein NMR spectroscopy (Academic Press, San Diego, California; 1996).
Brunger, A.T. X-PLOR (Yale University, New Haven, Connecticut; 1992 ).
Kuszewski, J., Gronenborn, A.M. & Clore, G.M. Protein Sci. 5, 1067–1080 (1996).
Thompson, J.D., Higgins, D.G. & Gibson, T.J. Nucleic Acids Res. 22, 4673–4680 (1994).
Retief, J.D. In Bioinformatics methods and protocols, Vol. 132 (eds, Misener, S. & Krawetz, S.A.) 243 (Humana Press Inc., Totowa; 1999 ).
Dayhoff, M.O. Atlas of protein sequence and structure (National Biomedical Research foundation, Washington, DC; 1979).
Rudolph, M.J., Wuebbens, M.M., Rajagopalan, K.V. & Schindelin, H. Nature Struct. Biol. 8, 42– 46 (2000).
Acknowledgements
We thank P.A. Karplus, R.J. MacIntyre, R.E. Oswald and R.-H. Chen for very helpful comments and discussion, L.E. Kay for use of his pulse sequence library, and F. Delaglio and D. Garrett (NIH/NIDDK) for use of their software tools. This research was funded by the NIH and the NSF. C.Wang was supported by a graduate fellowship from the Olin Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, C., Xi, J., Begley, T. et al. Solution structure of ThiS and implications for the evolutionary roots of ubiquitin. Nat Struct Mol Biol 8, 47–51 (2001). https://doi.org/10.1038/83041
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/83041
This article is cited by
-
Evolution of the archaeal and mammalian information processing systems: towards an archaeal model for human disease
Cellular and Molecular Life Sciences (2017)
-
Involvement of a eukaryotic-like ubiquitin-related modifier in the proteasome pathway of the archaeon Sulfolobus acidocaldarius
Nature Communications (2015)
-
Macromolecular juggling by ubiquitylation enzymes
BMC Biology (2013)
-
Synthetic biology approach to reconstituting the ubiquitylation cascade in bacteria
The EMBO Journal (2012)
-
Nmag_2608, an extracellular ubiquitin-like domain-containing protein from the haloalkaliphilic archaeon Natrialba magadii
Extremophiles (2012)