Key Points
-
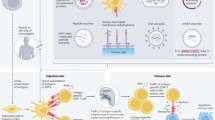
Personalized peptide vaccines (PPVs) are an immunotherapeutic approach based on the administration of multiple cancer peptides selected from a list of candidates to complement the pre-existing immunity of a patient
-
Vaccination with an appropriately individualized selection of peptides induces stronger and more rapid antitumour immunity than inoculation of conventional peptide vaccines
-
Early-phase trials demonstrated safety and efficacy of PPVs in patients with urological cancers and randomized trials showed significant survival improvements in patients with castration-resistant prostate cancer
-
Early-phase prostate cancer trials indicate that PPV administration at an early disease stage results in increased benefits, possibly owing to limited secretion of immunosuppressive cytokines and sufficient time to develop antitumour responses in this setting
-
Combination treatment employing PPVs with chemotherapeutic agents, radiotherapy, cryoablation, or checkpoint inhibitors might be an attractive future strategy, resulting in enhanced effects owing to changes in the local immune environment
-
Future studies need to evaluate the utility of PPVs in bladder and kidney cancer, determine means to select patients who will benefit most from this treatment and optimize protocols for combination therapies involving PPVs
Abstract
Immunotherapy is an important therapeutic modality for urological cancers and several immunological agents for their treatment, such as sipuleucel-T and immune checkpoint inhibitors, have been approved by the FDA. Personalized peptide vaccines (PPVs) are an immunotherapy that uses multiple cancer peptides that are selected to complement pre-existing host immunity. Vaccination with an appropriate, individualized selection of peptides, chosen from a list of candidates, induces stronger and more rapid antitumour immunity in comparison with inoculation of conventional peptide vaccines. Phase I and phase II trials have shown that PPVs are safe and effective in urological cancers. Randomized trials in patients with castration-resistant prostate cancer showed that PPVs can significantly improve progression-free survival and overall survival. However, further studies are needed to evaluate the utility of PPVs in other urological cancers, to identify those patients who will derive the greatest benefit from this approach and to optimize the protocols for combination therapies involving PPVs.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Shelley, M. D. et al. A systematic review of intravesical bacillus Calmette-Guerin plus transurethral resection versus transurethral resection alone in Ta and T1 bladder cancer. BJU Int. 88, 209–216 (2001).
Kantoff, P. W. et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 363, 411–422 (2010).
Motzer, R. J. et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N. Engl. J. Med. 373, 1803–1813 (2015).
Rosenberg, J. E. et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 387, 1909–1920 (2016).
Sasada, T., Noguchi, M., Yamada, A. & Itoh, K. Personalized peptide vaccination: a novel immunotherapeutic approach for advanced cancer. Hum. Vaccin. Immunother. 8, 1309–1313 (2012).
Noguchi, M. et al. A phase I study of personalized peptide vaccination using 14 kinds of vaccine in combination with low-dose estramustine in HLA-A24-positive patients with castration-resistant prostate cancer. Prostate 71, 470–479 (2011).
Uemura, H. et al. Immunological evaluation of personalized peptide vaccination monotherapy in patients with castration-resistant prostate cancer. Cancer Sci. 101, 601–608 (2010).
Noguchi, M. et al. A randomized phase II trial of personalized peptide vaccine plus low dose estramustine phosphate (EMP) versus standard dose EMP in patients with castration resistant prostate cancer. Cancer Immunol. Immunother. 59, 1001–1009 (2010).
Yoshimura, K. et al. A phase 2 randomized controlled trial of personalized peptide vaccine immunotherapy with low-dose dexamethasone versus dexamethasone alone in chemotherapy-naive castration-resistant prostate cancer. Eur. Urol. 70, 25–41 (2016).
Noguchi, M. et al. An open-label, randomized phase II trial of personalized peptide vaccination in patients with bladder cancer that progressed after platinum-based chemotherapy. Clin. Cancer Res. 22, 54–60 (2016).
Suekane, S. et al. Phase I trial of personalized peptide vaccination for cytokine-refractory metastatic renal cell carcinoma patients. Cancer Sci. 98, 1965–1968 (2007).
Negrier, S. et al. Recombinant human interleukin-2, recombinant human interferon alfa-2a, or both in metastatic renal-cell carcinoma. Groupe Francais d'Immunotherapie. N. Engl. J. Med. 338, 1272–1278 (1998).
McDermott, D. F. et al. Randomized phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. J. Clin. Oncol. 23, 133–141 (2005).
Rhodes, D. R., Barrette, T. R., Rubin, M. A., Ghosh, D. & Chinnaiyan, A. M. Meta-analysis of microarrays: interstudy validation of gene expression profiles reveals pathway dysregulation in prostate cancer. Cancer Res. 62, 4427–4433 (2002).
Sato, E. et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc. Natl Acad. Sci. USA 102, 18538–18543 (2005).
Schlom, J., Arlen, P. M. & Gulley, J. L. Cancer vaccines: moving beyond current paradigms. Clin. Cancer Res. 13, 3776–3782 (2007).
Harty, J. T. & Badovinac, V. P. Shaping and reshaping CD8+ T-cell memory. Nat. Rev. Immunol. 8, 107–119 (2008).
Gulley, J. L. & Drake, C. G. Immunotherapy for prostate cancer: recent advances, lessons learned, and areas for further research. Clin. Cancer Res. 17, 3884–3891 (2011).
Schreiber, R. D., Old, L. J. & Smyth, M. J. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 331, 1565–1570 (2011).
Melero, I. et al. Therapeutic vaccines for cancer: an overview of clinical trials. Nat. Rev. Clin. Oncol. 11, 509–524 (2014).
van der Burg, S. H., Arens, R., Ossendorp, F., van Hall, T. & Melief, C. J. Vaccines for established cancer: overcoming the challenges posed by immune evasion. Nat. Rev. Cancer 16, 219–233 (2016).
Gubin, M. M., Artyomov, M. N., Mardis, E. R. & Schreiber, R. D. Tumor neoantigens: building a framework for personalized cancer immunotherapy. J. Clin. Invest. 125, 3413–3421 (2015).
Itoh, K. & Yamada, A. Personalized peptide vaccines: a new therapeutic modality for cancer. Cancer Sci. 97, 970–976 (2006).
Hirayama, M. & Nishimura, Y. The present status and future prospects of peptide-based cancer vaccines. Int. Immunol. 28, 319–328 (2016).
Janssen, E. M. et al. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature 421, 852–856 (2003).
Kennedy, R. & Celis, E. T helper lymphocytes rescue CTL from activation-induced cell death. J. Immunol. 177, 2862–2872 (2006).
Chamoto, K. et al. Potentiation of tumor eradication by adoptive immunotherapy with T-cell receptor gene-transduced T-helper type 1 cells. Cancer Res. 64, 386–390 (2004).
Braumuller, H. et al. T-Helper-1-cell cytokines drive cancer into senescence. Nature 494, 361–365 (2013).
Bos, R. & Sherman, L. A. CD4+ T-cell help in the tumor milieu is required for recruitment and cytolytic function of CD8+ T lymphocytes. Cancer Res. 70, 8368–8377 (2010).
Melief, C. J. & van der Burg, S. H. Immunotherapy of established (pre)malignant disease by synthetic long peptide vaccines. Nat. Rev. Cancer 8, 351–360 (2008).
Banchereau, J. & Palucka, A. K. Dendritic cells as therapeutic vaccines against cancer. Nat. Rev. Immunol. 5, 296–306 (2005).
Palucka, K. & Banchereau, J. Cancer immunotherapy via dendritic cells. Nat. Rev. Cancer 12, 265–277 (2012).
Constantino, J., Gomes, C., Falcao, A., Cruz, M. T. & Neves, B. M. Antitumor dendritic cell-based vaccines: lessons from 20 years of clinical trials and future perspectives. Transl Res. 168, 74–95 (2016).
Drake, C. G. Update on prostate cancer vaccines. Cancer J. 17, 294–299 (2011).
Gohara, R. et al. Phase 1 clinical study of cyclophilin B peptide vaccine for patients with lung cancer. J. Immunother. 25, 439–444 (2002).
Chen, W. & McCluskey, J. Immunodominance and immunodomination: critical factors in developing effective CD8+ T-cell-based cancer vaccines. Adv. Cancer Res. 95, 203–247 (2006).
Stone, J. D., Harris, D. T. & Kranz, D. M. TCR affinity for p/MHC formed by tumor antigens that are self-proteins: impact on efficacy and toxicity. Curr. Opin. Immunol. 33, 16–22 (2015).
Lennerz, V. et al. The response of autologous T cells to a human melanoma is dominated by mutated neoantigens. Proc. Natl Acad. Sci. USA 102, 16013–16018 (2005).
Bobisse, S., Foukas, P. G., Coukos, G. & Harari, A. Neoantigen-based cancer immunotherapy. Ann. Transl Med. 4, 262 (2016).
Robbins, P. F. et al. Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nat. Med. 19, 747–752 (2013).
van Rooij, N. et al. Tumor exome analysis reveals neoantigen-specific T-cell reactivity in an ipilimumab-responsive melanoma. J. Clin. Oncol. 31, e439–e442 (2013).
Wick, D. A. et al. Surveillance of the tumor mutanome by T cells during progression from primary to recurrent ovarian cancer. Clin. Cancer Res. 20, 1125–1134 (2014).
Noguchi, M. et al. Induction of cellular and humoral immune responses to tumor cells and peptides in HLA-A24 positive hormone-refractory prostate cancer patients by peptide vaccination. Prostate 57, 80–92 (2003).
Komatsu, N., Shichijo, S., Nakagawa, M. & Itoh, K. New multiplexed flow cytometric assay to measure anti-peptide antibody: a novel tool for monitoring immune responses to peptides used for immunization. Scand. J. Clin. Lab. Invest. 64, 535–545 (2004).
Melief, C. J., van Hall, T., Arens, R., Ossendorp, F. & van der Burg, S. H. Therapeutic cancer vaccines. J. Clin. Invest. 125, 3401–3412 (2015).
Feyerabend, S. et al. Novel multi-peptide vaccination in Hla-A2+ hormone sensitive patients with biochemical relapse of prostate cancer. Prostate 69, 917–927 (2009).
Noguchi, M. et al. Immunological evaluation of individualized peptide vaccination with a low dose of estramustine for HLA-A24+ HRPC patients. Prostate 63, 1–12 (2005).
Noguchi, M. et al. A randomized phase II clinical trial of personalized peptide vaccination with metronomic low-dose cyclophosphamide in patients with metastatic castration-resistant prostate cancer. Cancer Immunol. Immunother. 65, 151–160 (2016).
UMIN Clinical Trials Registry. Umin.ac.jp https://upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000013251 (2016).
UMIN Clinical Trials Registry. Umin.ac.jp https://upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000012911 (2015).
UMIN Clinical Trials Registry. Umin.ac.jp https://upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000012051 (2015).
National Comprehensive Cancer Network. NCCN guidelines on prostate cancer, version 2.2017. NCCN http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf (2017).
Drake, C. G., Sharma, P. & Gerritsen, W. Metastatic castration-resistant prostate cancer: new therapies, novel combination strategies and implications for immunotherapy. Oncogene 33, 5053–5064 (2014).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02107391 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02020070 (2016).
Fong, L. et al. Potentiating endogenous antitumor immunity to prostate cancer through combination immunotherapy with CTLA4 blockade and GM-CSF. Cancer Res. 69, 609–615 (2009).
van den Eertwegh, A. J. et al. Combined immunotherapy with granulocyte-macrophage colony-stimulating factor-transduced allogeneic prostate cancer cells and ipilimumab in patients with metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol. 13, 509–517 (2012).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01322490 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01706458 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01420965 (2016).
Itoh, K., Platsoucas, C. D. & Balch, C. M. Autologous tumor-specific cytotoxic T lymphocytes in the infiltrate of human metastatic melanomas. Activation by interleukin 2 and autologous tumor cells, and involvement of the T cell receptor. J. Exp. Med. 168, 1419–1441 (1988).
Hong, M. et al. Chemotherapy induces intratumoral expression of chemokines in cutaneous melanoma, favoring T-cell infiltration and tumor control. Cancer Res. 71, 6997–7009 (2011).
Welters, M. J. et al. Vaccination during myeloid cell depletion by cancer chemotherapy fosters robust T cell responses. Sci. Transl Med. 8, 334ra52 (2016).
Kodumudi, K. N. et al. A novel chemoimmunomodulating property of docetaxel: suppression of myeloid-derived suppressor cells in tumor bearers. Clin. Cancer Res. 16, 4583–4594 (2010).
Zitvogel, L. et al. The anticancer immune response: indispensable for therapeutic success? J. Clin. Invest. 118, 1991–2001 (2008).
He, Q. et al. Low-dose paclitaxel enhances the anti-tumor efficacy of GM-CSF surface-modified whole-tumor-cell vaccine in mouse model of prostate cancer. Cancer Immunol. Immunother. 60, 715–730 (2011).
Naito, M. et al. Dexamethasone did not suppress immune boosting by personalized peptide vaccination for advanced prostate cancer patients. Prostate 68, 1753–1762 (2008).
Amin, A. et al. Survival with AGS-003, an autologous dendritic cell-based immunotherapy, in combination with sunitinib in unfavorable risk patients with advanced renal cell carcinoma (RCC): phase 2 study results. J. Immunother. Cancer 3, 14 (2015).
Madan, R. A., Gulley, J. L., Fojo, T. & Dahut, W. L. Therapeutic cancer vaccines in prostate cancer: the paradox of improved survival without changes in time to progression. Oncologist 15, 969–975 (2010).
Hoos, A. et al. Improved endpoints for cancer immunotherapy trials. J. Natl Cancer Inst. 102, 1388–1397 (2010).
Wolchok, J. D. et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin. Cancer Res. 15, 7412–7420 (2009).
Joniau, S. et al. Current vaccination strategies for prostate cancer. Eur. Urol. 61, 290–306 (2012).
Noguchi, M. et al. A phase II trial of personalized peptide vaccination in castration-resistant prostate cancer patients: prolongation of prostate-specific antigen doubling time. BMC Cancer 13, 613 (2013).
Noguchi, M. et al. Assessment of immunological biomarkers in patients with advanced cancer treated by personalized peptide vaccination. Cancer Biol. Ther. 10, 1266–1279 (2010).
Sheikh, N. A. et al. Sipuleucel-T immune parameters correlate with survival: an analysis of the randomized phase 3 clinical trials in men with castration-resistant prostate cancer. Cancer Immunol. Immunother. 62, 137–147 (2013).
Kirner, A., Mayer-Mokler, A. & Reinhardt, C. IMA901: a multi-peptide cancer vaccine for treatment of renal cell cancer. Hum. Vaccin. Immunother. 10, 3179–3189 (2014).
Rini, B. I. et al. IMA901, a multipeptide cancer vaccine, plus sunitinib versus sunitinib alone, as first-line therapy for advanced or metastatic renal cell carcinoma (IMPRINT): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 17, 1599–1611 (2016).
Noguchi, M. et al. Immunological evaluation of neoadjuvant peptide vaccination before radical prostatectomy for patients with localized prostate cancer. Prostate 67, 933–942 (2007).
Efstathiou, E. et al. Molecular characterization of enzalutamide-treated bone metastatic castration-resistant prostate cancer. Eur. Urol. 67, 53–60 (2015).
Efstathiou, E. et al. Effects of abiraterone acetate on androgen signaling in castrate-resistant prostate cancer in bone. J. Clin. Oncol. 30, 637–643 (2012).
Noguchi, M. et al. Phase I trial of a cancer vaccine consisting of 20 mixed peptides in patients with castration-resistant prostate cancer: dose-related immune boosting and suppression. Cancer Immunol. Immunother. 64, 493–505 (2015).
Walter, S. et al. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat. Med. 18, 1254–1261 (2012).
Kalbasi, A., June, C. H., Haas, N. & Vapiwala, N. Radiation and immunotherapy: a synergistic combination. J. Clin. Invest. 123, 2756–2763 (2013).
Finkelstein, S. E. et al. The confluence of stereotactic ablative radiotherapy and tumor immunology. Clin. Dev. Immunol. 2011, 439752 (2011).
Finkelstein, S. E. et al. Serial assessment of lymphocytes and apoptosis in the prostate during coordinated intraprostatic dendritic cell injection and radiotherapy. Immunotherapy 4, 373–382 (2012).
Sidana, A. Cancer immunotherapy using tumor cryoablation. Immunotherapy 6, 85–93 (2014).
Author information
Authors and Affiliations
Contributions
T.K. researched data and wrote the article. S.E. and H.U. reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
S.E. is a paid consultant/adviser to Astellas, AstraZeneca, and Takeda. H.U. is a paid consultant/adviser to AstraZeneca and Takeda. T.K. declares no competing interests.
Rights and permissions
About this article
Cite this article
Kimura, T., Egawa, S. & Uemura, H. Personalized peptide vaccines and their relation to other therapies in urological cancer. Nat Rev Urol 14, 501–510 (2017). https://doi.org/10.1038/nrurol.2017.77
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrurol.2017.77
This article is cited by
-
3D tumor spheroid microarray for high-throughput, high-content natural killer cell-mediated cytotoxicity
Communications Biology (2021)
-
Protective CD8+ T-cell response against Hantaan virus infection induced by immunization with designed linear multi-epitope peptides in HLA-A2.1/Kb transgenic mice
Virology Journal (2020)
-
New insights into the epigenetics of inflammatory rheumatic diseases
Nature Reviews Rheumatology (2017)
-
Personalized ex vivo multiple peptide enrichment and detection of T cells reactive to multiple tumor-associated antigens in prostate cancer patients
Medical Oncology (2017)