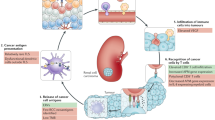
Abstract
Immunotherapy has been used in localized urothelial carcinoma for decades, especially in the treatment of superficial disease, in which instillation of BCG is a commonly used treatment option. Clinical investigations based on new insights into the immunogenic potential of metastatic urothelial carcinoma have led to the accelerated FDA approval of the immune checkpoint inhibitors atezolizumab, nivolumab, durvalumab, avelumab, and pembrolizumab. Preliminary findings suggest additional benefits of combinations of immunotherapeutic agents as a future treatment approach in metastatic urothelial carcinoma. Treatment experience with immunotherapy suggests that these drugs are associated with a unique spectrum of immune-related adverse events and specific immune-related patterns of response, including cases of pseudoprogression, which could impede the optimal use of immune checkpoint inhibitors in the clinic. Appropriate management of immune-related adverse events and a greater awareness of immune-mediated response patterns will help to inform treatment decisions and improve patient outcomes; predictive biomarkers of response might facilitate selection of patients who are most likely to respond to and benefit from these exciting new treatments.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Fleshner, N. E. et al. The national cancer data base report on bladder carcinoma. The American College of Surgeons commission on cancer and the American Cancer Society. Cancer 78, 1505–1513 (1996).
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2017. CA Cancer J. Clin. 67, 7–30 (2017).
National Cancer Institute. Cancer stat facts: bladder cancer. National Cancer Institute http://seer.cancer.gov/statfacts/html/urinb.html (2016).
Logothetis, C. J. et al. A prospective randomized trial comparing MVAC and CISCA chemotherapy for patients with metastatic urothelial tumors. J. Clin. Oncol. 8, 1050–1055 (1990).
Sternberg, C. N. et al. Randomized phase III trial of high-dose-intensity methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) chemotherapy and recombinant human granulocyte colony-stimulating factor versus classic MVAC in advanced urothelial tract tumors: European Organization for Research and Treatment of Cancer Protocol no. 30924. J. Clin. Oncol. 19, 2638–2646 (2001).
van de Putte, E. E. et al. Neoadjuvant induction dose-dense MVAC for muscle invasive bladder cancer: efficacy and safety compared with classic MVAC and gemcitabine/cisplatin. World J. Urol. 34, 157–162 (2016).
Dash, A. et al. Impact of renal impairment on eligibility for adjuvant cisplatin-based chemotherapy in patients with urothelial carcinoma of the bladder. Cancer 107, 506–513 (2006).
Sonpavde, G., Galsky, M. D., Latini, D. & Chen, G. J. Cisplatin-ineligible and chemotherapy-ineligible patients should be the focus of new drug development in patients with advanced bladder cancer. Clin. Genitourin. Cancer 12, 71–73 (2014).
Bellmunt, J. et al. Randomized phase III study comparing paclitaxel/cisplatin/gemcitabine and gemcitabine/cisplatin in patients with locally advanced or metastatic urothelial cancer without prior systemic therapy: EORTC intergroup study 30987. J. Clin. Oncol. 30, 1107–1113 (2012).
von der Maase, H. et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J. Clin. Oncol. 23, 4602–4608 (2005).
McCaffrey, J. A. et al. Phase II trial of docetaxel in patients with advanced or metastatic transitional-cell carcinoma. J. Clin. Oncol. 15, 1853–1857 (1997).
Bellmunt, J. et al. Long-term survival results of a randomized phase III trial of vinflunine plus best supportive care versus best supportive care alone in advanced urothelial carcinoma patients after failure of platinum-based chemotherapy. Ann. Oncol. 24, 1466–1472 (2013).
Sharma, P. et al. CD8 tumor-infiltrating lymphocytes are predictive of survival in muscle-invasive urothelial carcinoma. Proc. Natl Acad. Sci. USA 104, 3967–3972 (2007).
Lawrence, M. S. et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 499, 214–218 (2013).
The Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 507, 315–322 (2014).
Redelman-Sidi, G., Glickman, M. S. & Bochner, B. H. The mechanism of action of BCG therapy for bladder cancer-a current perspective. Nat. Rev. Urol. 11, 153–162 (2014).
Clark, P. E. et al. Bladder cancer. J. Natl Compr. Canc. Netw. 11, 446–475 (2013).
Lam, J. S. et al. Bacillus calmete-guerin plus interferon-α2B intravesical therapy maintains an extended treatment plan for superficial bladder cancer with minimal toxicity. Urol. Oncol. 21, 354–360 (2003).
Siefker-Radtke, A. O. et al. Phase III trial of fluorouracil, interferon alpha-2b, and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in metastatic or unresectable urothelial cancer. J. Clin. Oncol. 20, 1361–1367 (2002).
Chou, R. et al. Intravesical therapy for the treatment of nonmuscle invasive bladder cancer: a systematic review and meta-analysis. J. Urol. 197, 1189–1199 (2017).
Faraj, S. F. et al. Assessment of tumoral PD-L1 expression and intratumoral CD8+ T cells in urothelial carcinoma. Urology 85, 703–706 (2015).
Inman, B. A. et al. PD-L1 (B7-H1) expression by urothelial carcinoma of the bladder and BCG-induced granulomata: associations with localized stage progression. Cancer 109, 1499–1505 (2007).
Fife, B. T. & Bluestone, J. A. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol. Rev. 224, 166–182 (2008).
Finger, L. R. et al. The human PD-1 gene: complete cDNA, genomic organization, and developmentally regulated expression in B cell progenitors. Gene 197, 177–187 (1997).
Shi, L., Chen, S., Yang, L. & Li, Y. The role of PD-1 and PD-L1 in T-cell immune suppression in patients with hematological malignancies. J. Hematol. Oncol. 6, 74 (2013).
Thibult, M. L. et al. PD-1 is a novel regulator of human B-cell activation. Int. Immunol. 25, 129–137 (2013).
Zou, W., Wolchok, J. D. & Chen, L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci. Transl. Med. 8, 328rv4 (2016).
Bardhan, K., Anagnostou, T. & Boussiotis, V. A. The PD1:PD-L1/2 pathway from discovery to clinical implementation. Front. Immunol. 7, 550 (2016).
Dong, H. et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat. Med. 8, 793–800 (2002).
Latchman, Y. et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2, 261–268 (2001).
Agrawal, B., Gendler, S. J. & Longenecker, B. M. The biological role of mucins in cellular interactions and immune regulation: prospects for cancer immunotherapy. Mol. Med. Today 4, 397–403 (1998).
Driessens, G. et al. β-Catenin inhibits T cell activation by selective interference with linker for activation of T cells-phospholipase C-γ1 phosphorylation. J. Immunol. 186, 784–790 (2011).
Dunne, M. R. et al. HLA-DR expression in tumor epithelium is an independent prognostic indicator in esophageal adenocarcinoma patients. Cancer Immunol. Immunother. 66, 841–850 (2017).
Kouo, T. et al. Galectin-3 shapes antitumor immune responses by suppressing CD8+ T cells via LAG-3 and inhibiting expansion of plasmacytoid dendritic cells. Cancer Immunol. Res. 3, 412–423 (2015).
Pandolfi, F. et al. Expression of HLA-A2 antigen in human melanoma cell lines and its role in T-cell recognition. Cancer Res. 51, 3164–3170 (1991).
Rodriguez, P. C. et al. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. 64, 5839–5849 (2004).
Shan, Y. S. et al. Suppression of mucin 2 promotes interleukin-6 secretion and tumor growth in an orthotopic immune-competent colon cancer animal model. Oncol. Rep. 32, 2335–2342 (2014).
Sweis, R. F. et al. Molecular drivers of the non-T-cell-inflamed tumor microenvironment in urothelial bladder cancer. Cancer Immunol. Res. 4, 563–568 (2016).
Gabrilovich, D. I. & Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 9, 162–174 (2009).
Choueiri, T. K. et al. Immunomodulatory activity of nivolumab in metastatic renal cell carcinoma (mRCC): association of biomarkers with clinical outcomes [abstract]. J. Clin. Oncol. 33 (Suppl. 15), 4500 (2015).
Pardoll, D. M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264 (2012).
Koh, J. et al. Clinicopathologic analysis of programmed cell death-1 and programmed cell death-ligand 1 and 2 expressions in pulmonary adenocarcinoma: comparison with histology and driver oncogenic alteration status. Mod. Pathol. 28, 1154–1166 (2015).
Tarhini, A. A. et al. Immune monitoring of the circulation and the tumor microenvironment in patients with regionally advanced melanoma receiving neoadjuvant ipilimumab. PLoS ONE 9, e87705 (2014).
Carthon, B. C. et al. Preoperative CTLA-4 blockade: tolerability and immune monitoring in the setting of a presurgical clinical trial. Clin. Cancer Res. 16, 2861–2871 (2010).
Liakou, C. I. et al. CTLA-4 blockade increases IFNγ-producing CD4+ICOShi cells to shift the ratio of effector to regulatory T cells in cancer patients. Proc. Natl Acad. Sci. USA 105, 14987–14992 (2008).
Rosenberg, J. E. et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 387, 1909–1920 (2016).
Loriot, Y. et al. Atezolizumab in platinum-treated locally advanced or metastatic urothelial carcinoma (mUC): updated OS, safety and biomarkers from the phase II IMvigor210 study. Ann. Oncol. 27 (Suppl. 6), 783P (2016).
Balar, A. V. et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 389, 67–76 (2017).
Sharma, P. et al. Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (CheckMate 032): a multicentre, open-label, two-stage, multi-arm, phase 1/2 trial. Lancet Oncol. 17, 1590–1598 (2016).
Sharma, P. et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 389, 67–76 (2017).
Massard, C. et al. Safety and efficacy of durvalumab (MEDI4736), an anti-programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J. Clin. Oncol. 34, 3119–3125 (2016).
Plimack, E. R. et al. Pembrolizumab (MK-3475) for advanced urothelial cancer: updated results and biomarker analysis from KEYNOTE-012 [abstract]. J. Clin. Oncol. 33 (Suppl. 7), 4502 (2015).
Bellmunt, J. et al. KEYNOTE-045: randomized phase 3 trial of pembrolizumab (MK-3475) versus paclitaxel, docetaxel, or vinflunine for previously treated metastatic urothelial cancer [abstract]. J. Clin. Oncol. 33 (Suppl. 7), TPS4571 (2015).
Balar, A. et al. Pembrolizumab (pembro) as first-line therapy for advanced/unresectable or metastatic urothelial cancer: preliminary results from the phase 2 keynote-052 study. Ann. Oncol. 27 (Suppl. 6), LBA32_PR (2016).
Patel, M. et al. Avelumab (MSB0010718C; anti–PD-L1) in patients with metastatic urothelial carcinoma progressed after platinum-based therapy or platinum ineligible. Ann. Oncol. 27 (Suppl. 6), 777PD (2016).
Sharma, P. et al. Efficacy and safety of nivolumab plus ipilimumab in metastatic urothelial cancer: first results from the phase I/II checkmate 032 study society for immunotherapy of cancer (SITC) annual meeting. National Harbor (in the press).
Apolo, A. B. et al. A phase I study of cabozantinib plus nivolumab (CaboNivo) in patients (pts) refractory metastatic urothelial carcinoma (mUC) and other genitourinary (GU) tumors. Ann. Oncol. 27 (Suppl. 6), 774PD (2016).
Bristol-Myers Squibb. Latest News. Bristol-Myers Squibb https://investors.bms.com/iframes/press-releases/press-release-details/2016/Bristol-Myers-Squibb-and-Calithera-Biosciences-Announce-Clinical-Collaboration-to-Evaluate-Opdivo-nivolumab-in-Combination-with-CB-839-in-Clear-Cell-Renal-Cell-Carcinoma/default.aspx (2016).
Powles, T. et al. A phase 3 study of first-line durvalumab (MEDI4736) ± tremelimumab versus standard of care (SoC) chemotherapy (CT) in patients (pts) with unresectable Stage IV urothelial bladder cancer (UBC): DANUBE [abstract]. J. Clin. Oncol. 34 (Suppl. 15), TPS4574 (2016).
Hamid, O. et al. Combination of an anti-PD-1 antibody, with durvalumab, an anti-PD-L1 antibody: a phase 1, open-label study in advanced malignancies. Ann. Oncol. 27 (Suppl. 6), 1050PD (2016).
Lee, H. T. et al. Molecular mechanism of PD-1/PD-L1 blockade via anti-PD-L1 antibodies atezolizumab and durvalumab. Sci. Rep. 7, 5532 (2017).
Powles, T. et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 515, 558–562 (2014).
Genetech. TECENTRIQ prescribing information. Genetech https://www.gene.com/download/pdf/tecentriq_prescribing.pdf (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02302807?term=NCT02302807&rank=1 (2017).
Roche. Roche provides update on phase III study of TECENTRIQ® (atezolizumab) in people with previously treated advanced bladder cancer. Roche http://www.roche.com/media/store/releases/med-cor-2017-05-10.htm (2017).
Wang, C. et al. In vitro characterization of the anti-PD-1 antibody nivolumab, BMS-936558, and in vivo toxicology in non-human primates. Cancer Immunol. Res. 2, 846–856 (2014).
Bristol-Myers Squibb. OPDIVO (nivolumab) prescribing information. Bristol-Myers Squibb https://packageinserts.bms.com/pi/pi_opdivo.pdf (2017).
Robert, C. et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 372, 320–330 (2015).
Borghaei, H. et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N. Engl. J. Med. 373, 1627–1639 (2015).
Brahmer, J. et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N. Engl. J. Med. 373, 123–135 (2015).
[No authors listed.] Nivolumab doubles survival for patients with HNSCC. Cancer Discov. 6, OF3 (2016).
Motzer, R. J. et al. Nivolumab for metastatic renal cell carcinoma: results of a randomized phase II trial. J. Clin. Oncol. 33, 1430–1437 (2015).
Ansell, S. M. et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N. Engl. J. Med. 372, 311–319 (2015).
Overman, M. J. et al. Nivolumab ± ipilimumab in treatment (tx) of patients (pts) with metastatic colorectal cancer (mCRC) with and without high microsatellite instability (MSI-H): CheckMate-142 interim results [abstract]. J. Clin. Oncol. 34 (Suppl. 15), 3501 (2016).
El-Khoueiry, A. B. et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 389, 2492–2502 (2017).
US Food and Drug Administration. Imfinzi (durvalumab) prescribing information. FDA https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761069s000lbl.pdf (2017).
Apolo, A. B. et al. Avelumab, an anti-programmed death-ligand 1 antibody, in patients with refractory metastatic urothelial carcinoma: results from a multicenter, phase Ib study. J. Clin. Oncol. 35, 2117–2124 (2017).
US Food and Drug Administration. Bavencio (avelumab) prescribing information. FDA https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761049s000lbl.pdf (2017).
Boyerinas, B. et al. Antibody-dependent cellular cytotoxicity activity of a novel anti-PD-L1 antibody avelumab (MSB0010718C) on human tumor cells. Cancer Immunol. Res. 3, 1148–1157 (2015).
Bellmunt, J. et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N. Engl. J. Med. 376, 1015–1026 (2017).
Merck. Keytruda (pembrolizumab) prescribing information. Merck https://www.merck.com/product/usa/pi_circulars/k/keytruda/keytruda_pi.pdf (2017).
Larkin, J. et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 373, 23–34 (2015).
Antonia, S. et al. Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicentre, phase 1b study. Lancet Oncol. 17, 299–308 (2016).
Hammers, H. J. et al. Updated results from a phase I study of nivolumab (Nivo) in combination with ipilimumab (Ipi) in metastatic renal cell carcinoma (mRCC): the CheckMate 016 study. J. Clin. Oncol. http://dx.doi.org/10.1200/JCO.2016.72.1985 (2017).
Wolchok, J. D. et al. Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 369, 122–133 (2013).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03036098 (2017).
Hoimes, C. J. et al. Hcrn GU14-188: neoadjuvant pembrolizumab (P) and gemcitabine (G) with or without cisplatin (C) in muscle invasive urothelial cancer (MIUC) [abstract]. J. Clin. Oncol. 34 (Suppl. 15), TPS4578 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02178722 (2017).
Weber, J. S., Kahler, K. C. & Hauschild, A. Management of immune-related adverse events and kinetics of response with ipilimumab. J. Clin. Oncol. 30, 2691–2697 (2012).
Weber, J. S. et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J. Clin. Oncol. 35, 785–792 (2017).
Medina, P. J. & Adams, V. R. PD-1 pathway inhibitors: Immuno-oncology agents for restoring antitumor immune responses. Pharmacotherapy 36, 317–334 (2016).
Naidoo, J. et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann. Oncol. 26, 2375–2391 (2015).
Dubin, K. et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat. Commun. 7, 10391 (2016).
Villadolid, J. & Amin, A. Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities. Transl Lung Cancer Res. 4, 560–575 (2015).
Weber, J. S., Postow, M., Lao, C. D. & Schadendorf, D. Management of adverse events following treatment with anti-programmed death-1 agents. Oncologist 21, 1230–1240 (2016).
Mancini, S., Amorotti, E., Vecchio, S., Ponz de Leon, M. & Roncucci, L. Infliximab-related hepatitis: discussion of a case and review of the literature. Intern. Emerg. Med. 5, 193–200 (2010).
Merrill, S. P. et al. Early administration of infliximab for severe ipilimumab-related diarrhea in a critically ill patient. Ann. Pharmacother. 48, 806–810 (2014).
Pages, C. et al. Ipilimumab-induced acute severe colitis treated by infliximab. Melanoma Res. 23, 227–230 (2013).
Kim, C., Gao, J., Shannon, V. R. & Siefker-Radtke, A. Systemic sarcoidosis first manifesting in a tattoo in the setting of immune checkpoint inhibition. BMJ Case Rep. http://dx.doi.org/10.1136/bcr-2016-216217 (2016).
Escudier, B. et al. Treatment beyond progression in patients with advanced renal cell carcinoma treated with nivolumab in CheckMate 025. Eur. Urol. 72, 368–376 (2017).
Wolchok, J. D. et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin. Cancer Res. 15, 7412–7420 (2009).
Hodi, F. S. et al. Evaluation of immune-related response criteria and RECIST v1.1 in patients with advanced melanoma treated with pembrolizumab. J. Clin. Oncol. 34, 1510–1517 (2016).
Motzer, R. J. et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N. Engl. J. Med. 373, 1803–1813 (2015).
Chiou, V. L. & Burotto, M. Pseudoprogression and immune-related response in solid tumors. J. Clin. Oncol. 33, 3541–3543 (2015).
Imafuku, K., Hata, H., Kitamura, S., Yanagi, T. & Shimizu, H. Ultrasonographic findings can identify 'pseudoprogression' under nivolumab therapy. Br. J. Dermatol. http://dx.doi.org/10.1111/bjd.15198 (2016).
Champiat, S. et al. Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin. Cancer Res. 23, 1920–1928 (2017).
Saada-Bouzid, E. et al. Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann. Oncol. 28, 1605–1611 (2017).
Kato, S. et al. Hyperprogressors after immunotherapy: analysis of genomic alterations associated with accelerated growth rate. Clin. Cancer Res. 23, 4242–4250 (2017).
McLaughlin, J. et al. Quantitative assessment of the heterogeneity of PD-L1 expression in non-small-cell lung cancer. JAMA Oncol. 2, 46–54 (2016).
Callea, M. et al. Differential expression of PD-L1 between primary and metastatic sites in clear-cell renal cell carcinoma. Cancer Immunol. Res. 3, 1158–1164 (2015).
Chalmers, Z. R. et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 9, 34 (2017).
Choi, W. et al. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell 25, 152–165 (2014).
Bahce, I. et al. Personalizing NSCLC therapy by characterizing tumors using TKI-PET and immuno-PET. Lung Cancer 107, 1–13 (2017).
Kikuchi, M. et al. Preclinical immunoPET/CT imaging using Zr-89-labeled anti-PD-L1 monoclonal antibody for assessing radiation-induced PD-L1 upregulation in head and neck cancer and melanoma. Oncoimmunology 6, e1329071 (2017).
McConkey, D. J. et al. A prognostic gene expression signature in the molecular classification of chemotherapy-naive urothelial cancer is predictive of clinical outcomes from neoadjuvant chemotherapy: a phase 2 trial of dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin with bevacizumab in urothelial cancer. Eur. Urol. 69, 855–862 (2016).
Acknowledgements
The authors thank M. Soushko and L. Hargett for editorial assistance, funded by Bristol-Myers Squibb.
Author information
Authors and Affiliations
Contributions
Both authors researched data for the article, made substantial contributions to discussions of content, wrote the article, and reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
A.S.-R. declares associations with AstraZeneca, Bristol-Myers Squibb (BMS), Eisai, EMD Serono, Genentech, Janssen, Merck, Threshold Pharmaceuticals, Inovio, and Vertex as a member of the scientific advisory boards and has acted as a speaker and preceptor for Genentech. B.C. declares receipt of honoraria from Prometheus Pharmaceuticals; research funding from BMS, MedImmune, and Prometheus Pharmaceuticals; and funding for travel expenses from BMS, MedImmune, and Prometheus Pharmaceuticals.
Rights and permissions
About this article
Cite this article
Siefker-Radtke, A., Curti, B. Immunotherapy in metastatic urothelial carcinoma: focus on immune checkpoint inhibition. Nat Rev Urol 15, 112–124 (2018). https://doi.org/10.1038/nrurol.2017.190
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrurol.2017.190
This article is cited by
-
Clinical significance of blocking novel immune checkpoint B7-H4 in urothelial carcinoma of bladder as a potential therapeutic target
Medical Oncology (2024)
-
Nano-immunotherapy: overcoming delivery challenge of immune checkpoint therapy
Journal of Nanobiotechnology (2023)
-
A robust gene prognostic index composed of GZMB, IRF1, and TP63 can stratify the risk of two metastatic urothelial carcinoma cohorts based on immune checkpoint blockade therapy
Journal of Cancer Research and Clinical Oncology (2023)
-
The biology and rationale of targeting nectin-4 in urothelial carcinoma
Nature Reviews Urology (2021)
-
Investigation on potential biomarkers of hyperprogressive disease (HPD) triggered by immune checkpoint inhibitors (ICIs)
Clinical and Translational Oncology (2021)