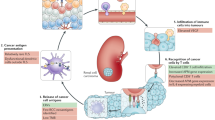
Abstract
An urgent need exists to improve the outcomes of patients with muscle-invasive bladder cancer (MIBC), and especially of those with metastatic disease. Treatments that enhance antitumour immune responses — such as immune-checkpoint inhibition — provide an opportunity to do this. Despite initial success, durable response rates in patients with advanced-stage MIBC treated with novel inhibitory antibodies targeting programmed cell death protein 1 (PD-1) or its endogenous ligand programmed cell death 1 ligand 1 (PD-L1) remain low. Radiotherapy is part of the management of bladder cancer in many patients. Evidence that radiotherapy has immunogenic properties is now available, but radiotherapy-induced immune responses are often negated by immunosuppression within the tumour microenvironment. Anti-PD-1 or anti-PD-L1 antibodies might enhance radiotherapy-induced antitumour immunity. This effect has been demonstrated in preclinical models of bladder cancer, and clinical trials involving this approach are currently recruiting. Combination treatment strategies provide an exciting opportunity for urological oncologists to not only improve the chances of cure in patients undergoing radical treatment for MIBC, but also to increase long-term response rates in those with metastatic disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Cancer Research UK. Bladder cancer statistics. CRUK www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/bladder-cancer (2014).
National Institute for Health and Care Excellence. Bladder cancer: diagnosis and management. NICE https://www.nice.org.uk/guidance/NG2 (2015).
Advanced Bladder Cancer Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: a systematic review and meta-analysis. Lancet 361, 1927–1934 (2003).
von der Maase, H. et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J. Clin. Oncol. 23, 4602–4608 (2005).
Bellmunt, J. et al. Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J. Clin. Oncol. 27, 4454–4461 (2009).
Apolo, A. B. et al. Updated efficacy and safety of avelumab in metastatic urothelial carcinoma (mUC): pooled analysis from 2 cohorts of the phase 1b Javelin solid tumor study. J. Clin. Oncol. 35 (Suppl. 15), 4528 (2017).
Balar, A. V. et al. Pembrolizumab as first-line therapy in cisplatin-ineligible advanced urothelial cancer: results from the total KEYNOTE-052 study population. J. Clin. Oncol. 35 (Suppl. 6), 284 (2017).
Balar, A. V. et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 389, 67–76 (2017).
Bellmunt, J. et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N. Engl. J. Med. 376, 1015–1026 (2017).
Hahn, N. M. et al. Updated efficacy and tolerability of durvalumab in locally advanced or metastatic urothelial carcinoma (UC). J. Clin. Oncol. 35 (Suppl. 15), 4525 (2017).
Rosenberg, J. E. et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 387, 1909–1920 (2016).
Sharma, P. et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 18, 312–322 (2017).
Burnette, B. C. et al. The efficacy of radiotherapy relies upon induction of type i interferon-dependent innate and adaptive immunity. Cancer Res. 71, 2488–2496 (2011).
Fuertes, M. B. et al. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. J. Exp. Med. 208, 2005–2016 (2011).
Gasser, S., Orsulic, S., Brown, E. J. & Raulet, D. H. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature 436, 1186–1190 (2005).
Gupta, A. et al. Radiotherapy promotes tumor-specific effector CD8+ T cells via dendritic cell activation. J. Immunol. 189, 558–566 (2012).
Obeid, M. et al. Calreticulin exposure is required for the immunogenicity of gamma-irradiation and UVC light-induced apoptosis. Cell Death Differ. 14, 1848–1850 (2007).
Reits, E. A. et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J. Exp. Med. 203, 1259–1271 (2006).
Stangl, S. et al. Detection of irradiation-induced, membrane heat shock protein 70 (Hsp70) in mouse tumors using Hsp70 Fab fragment. Radiother. Oncol. 99, 313–316 (2011).
Surace, L. et al. Complement is a central mediator of radiotherapy-induced tumor-specific immunity and clinical response. Immunity 42, 767–777 (2015).
Suzuki, Y. et al. Immunogenic tumor cell death induced by chemoradiotherapy in patients with esophageal squamous cell carcinoma. Cancer Res. 72, 3967–3976 (2012).
Morales, A., Eidinger, D. & Bruce, A. W. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J. Urol. 116, 180–183 (1976).
Han, R. F. & Pan, J. G. Can intravesical bacillus Calmette-Guerin reduce recurrence in patients with superficial bladder cancer? A meta-analysis of randomized trials. Urology 67, 1216–1223 (2006).
Shelley, M. D. et al. A systematic review of intravesical bacillus Calmette-Guerin plus transurethral resection versus transurethral resection alone in Ta and T1 bladder cancer. BJU Int. 88, 209–216 (2001).
Sylvester, R. J., van der M. A. & Lamm, D. L. Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: a meta-analysis of the published results of randomized clinical trials. J. Urol. 168, 1964–1970 (2002).
Babjuk, M. et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder, the 2011 update. Eur. Urol. 59, 997–1008 (2011).
Redelman-Sidi, G., Glickman, M. S. & Bochner, B. H. The mechanism of action of BCG therapy for bladder cancer—a current perspective. Nat. Rev. Urol. 11, 153–162 (2014).
Kelley, D. R. et al. Prognostic value of purified protein derivative skin test and granuloma formation in patients treated with intravesical bacillus Calmette-Guerin. J. Urol. 135, 268–271 (1986).
Taniguchi, K. et al. Systemic immune response after intravesical instillation of bacille Calmette-Guérin (BCG) for superficial bladder cancer. Clin. Exp. Immunol. 115, 131–135 (1999).
Witjes, J. A. et al. EAU guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2013 guidelines. Eur. Urol. 65, 778–792 (2014).
Hall, E. J. & Giaccia, A. J. Radiobiology for the Radiologist 6th edn (Lippincott Williams & Wilkins, 2006).
Albert, M. L., Sauter, B. & Bhardwaj, N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature 392, 86–89 (1998).
Obeid, M. et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat. Med. 13, 54–61 (2007).
Kroemer, G., Galluzzi, L., Kepp, O. & Zitvogel, L. Immunogenic cell death in cancer therapy. Annu. Rev. Immunol. 31, 51–72 (2013).
Sellers, D. J. & McKay, N. Developments in the pharmacotherapy of the overactive bladder. Curr. Opin. Urol. 17, 223–230 (2007).
D'Eliseo, D., Manzi, L. & Velotti, F. Capsaicin as an inducer of damage-associated molecular patterns (DAMPs) of immunogenic cell death (ICD) in human bladder cancer cells. Cell Stress Chaperones. 18, 801–808 (2013).
Casares, N. et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J. Exp. Med. 202, 1691–1701 (2005).
Loehrer, P. J. et al. A randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J. Clin. Oncol. 10, 1066–1073 (1992).
Sternberg, C. N. et al. Randomized phase III trial of high-dose-intensity methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) chemotherapy and recombinant human granulocyte colony-stimulating factor versus classic MVAC in advanced urothelial tract tumors: European Organization for Research and Treatment of Cancer Protocol no. 30924. J. Clin. Oncol. 19, 2638–2646 (2001).
Kepp, O. et al. Consensus guidelines for the detection of immunogenic cell death. Oncoimmunology 3, e955691 (2014).
Cambier, S. et al. EORTC nomograms and risk groups for predicting recurrence, progression, and disease-specific and overall survival in non-muscle-invasive stage Ta-T1 urothelial bladder cancer patients treated with 1–3 years of maintenance Bacillus Calmette-Guérin. Eur. Urol. 69, 60–69 (2016).
Caffo, O. et al. Concurrent gemcitabine and radiotherapy for the treatment of muscle-invasive bladder cancer: a pooled individual data analysis of eight phase I-II trials. Radiother. Oncol. 21, 193–198 (2016).
James, N. D. et al. Radiotherapy with or without chemotherapy in muscle-invasive bladder cancer. N. Engl. J. Med. 366, 1477–1488 (2012).
Siva, S., MacManus, M. P., Martin, R. F. & Martin, O. A. Abscopal effects of radiation therapy: a clinical review for the radiobiologist. Cancer Lett. 356, 82–90 (2015).
Dunn, G. P., Old, L. J. & Schreiber, R. D. The three Es of cancer immunoediting. Annu. Rev. Immunol. 22, 329–360 (2004).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Curiel, T. J. et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 10, 942–949 (2004).
Lee, I. et al. Recruitment of Foxp3+ T regulatory cells mediating allograft tolerance depends on the CCR4 chemokine receptor. J. Exp. Med. 201, 1037–1044 (2005).
Noy, R. & Pollard, J. W. Tumor-associated macrophages: from mechanisms to therapy. Immunity 41, 49–61 (2014).
Kono, K. et al. CD4(+)CD25high regulatory T cells increase with tumor stage in patients with gastric and esophageal cancers. Cancer Immunol. Immunother. 55, 1064–1071 (2006).
Zhang, Q. W. et al. Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PLoS ONE 7, e50946 (2012).
Khanna, R. Tumour surveillance: missing peptides and MHC molecules. Immunol. Cell Biol. 76, 20–26 (1998).
Paulson, K. G. et al. Downregulation of MHC-I expression is prevalent but reversible in Merkel cell carcinoma. Cancer Immunol. Res. 2, 1071–1079 (2014).
Keir, M. E., Butte, M. J., Freeman, G. J. & Sharpe, A. H. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 26, 677–704 (2008).
Taube, J. M. et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci. Transl. Med. 4, 127ra137 (2012).
Brahmer, J. et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N. Engl. J. Med. 373, 123–135 (2015).
Motzer, R. J. et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N. Engl. J. Med. 373, 1803–1813 (2015).
Robert, C. et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 372, 320–330 (2015).
Roche. Media release. Roche http://www.roche.com/media/store/releases/med-cor-2017-05-10.htm (2017).
Heo, J. H. et al. Abstract A16: expression of PD-L1 and BCG immunotherapy in non-muscle invasive bladder cancer [abstract]. Cancer Res. 76, A16 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02324582 (2017).
Deng, L. et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J. Clin. Invest. 124, 687–695 (2014).
Dovedi, S. J. et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 74, 5458–5468 (2014).
Wu, C. T., Chen, W. C., Chang, Y. H., Lin, W. Y. & Chen, M. F. The role of PD-L1 in the radiation response and clinical outcome for bladder cancer. Sci. Rep. 6, 19740 (2016).
Chan, E. S. et al. Optimizing orthotopic bladder tumor implantation in a syngeneic mouse model. J. Urol. 182, 2926–2931 (2009).
Zhang, N., Li, D., Shao, J. & Wang, X. Animal models for bladder cancer: the model establishment and evaluation (review). Oncol. Lett. 9, 1515–1519 (2015).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02560636 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02621151 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02662062 (2017).
Hoskin, P. J., Rojas, A. M., Bentzen, S. M. & Saunders, M. I. Radiotherapy with concurrent carbogen and nicotinamide in bladder carcinoma. J. Clin. Oncol. 28, 4912–4918 (2010).
Murray, L. et al. Stereotactic ablative radiotherapy (SABR) in patients with medically inoperable peripheral early stage lung cancer: outcomes for the first UK SABR cohort. Clin. Oncol. (R. Coll. Radiol) 28, 4–12 (2016).
SABR UK Consortium. Stereotactic ablative body radiation therapy (SABR): a resource. Action Radiotherapy http://www.actionradiotherapy.org/wp-content/uploads/2016/02/UKSABRConsortiumGuidelinesv51.pdf (2016).
Filatenkov, A. et al. Ablative tumor radiation can change the tumor immune cell microenvironment to induce durable complete remissions. Clin. Cancer. Res. 21, 3727–3739 (2015).
Sharabi, A. B. et al. Stereotactic radiation therapy augments antigen-specific PD-1-mediated antitumor immune responses via cross-presentation of tumor antigen. Cancer Immunol. Res. 3, 345–355 (2015).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02826564 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02447549 (2017).
Søndergaard, J. et al. A comparison of morbidity following conformal versus intensity-modulated radiotherapy for urinary bladder cancer. Acta Oncol. 53, 1321–1328 (2014).
Robert, C. et al. Pembrolizumab versus ipilimumab in advanced melanoma. N. Engl. J. Med. 372, 2521–2532 (2015).
Liniker, E. et al. Activity and safety of radiotherapy with anti-PD-1 drug therapy in patients with metastatic melanoma. Oncoimmunology 5, e1214788 (2016).
Carbognin, L. et al. Differential activity of nivolumab, pembrolizumab and MPDL3280A according to the tumor expression of programmed death-ligand-1 (PD-L1): sensitivity analysis of trials in melanoma, lung and genitourinary cancers. PLoS ONE 10, e0130142 (2015).
Bellmunt, J. et al. Association of PD-L1 expression on tumor-infiltrating mononuclear cells and overall survival in patients with urothelial carcinoma. Ann. Oncol. 26, 812–817 (2015).
Schumacher, T. N. & Schreiber, R. D. Neoantigens in cancer immunotherapy. Science 348, 69–74 (2015).
Rizvi, N. A. et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348, 124–128 (2015).
The Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 507, 315–322 (2014).
Tokito, T. et al. Predictive relevance of PD-L1 expression combined with CD8+ TIL density in stage III non-small cell lung cancer patients receiving concurrent chemoradiotherapy. Eur. J. Cancer 55, 7–14 (2016).
Choudhury, A. et al. MRE11 expression is predictive of cause-specific survival following radical radiotherapy for muscle-invasive bladder cancer. Cancer Res. 70, 7017–7026 (2010).
Eustace, A. et al. Necrosis predicts benefit from hypoxia-modifying therapy in patients with high risk bladder cancer enrolled in a phase III randomised trial. Radiother. Oncol. 108, 40–47 (2013).
US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT03115801 (2017).
Acknowledgements
R.W. researched data for this article, R.W. and J.H. made a substantial contribution to discussions of content, R.W. wrote the manuscript, and J.H., T.I., and A.C. edited and/or reviewed the manuscript before submission.
Author information
Authors and Affiliations
Contributions
R.W. researched data for this article, R.W. and J.H. made a substantial contribution to discussions of content, R.W. wrote the manuscript, and J.H., T.I., and A.C. edited and/or reviewed the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Walshaw, R., Honeychurch, J., Illidge, T. et al. The anti-PD-1 era — an opportunity to enhance radiotherapy for patients with bladder cancer. Nat Rev Urol 15, 251–259 (2018). https://doi.org/10.1038/nrurol.2017.172
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrurol.2017.172
This article is cited by
-
GPR162 activates STING dependent DNA damage pathway as a novel tumor suppressor and radiation sensitizer
Signal Transduction and Targeted Therapy (2023)
-
Higher expression of programmed cell death 4 (PDCD4) in acute myeloid leukemia is associated with better prognosis after chemotherapy
Annals of Hematology (2023)
-
Distant metastasis without regional progression in non-muscle invasive bladder cancer: case report and pooled analysis of literature
World Journal of Surgical Oncology (2022)
-
Next-generation sequencing-guided molecular-targeted therapy and immunotherapy for biliary tract cancers
Cancer Immunology, Immunotherapy (2021)
-
Combined radiotherapy and immunotherapy in urothelial bladder cancer: harnessing the full potential of the anti-tumor immune response
World Journal of Urology (2021)