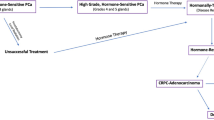
Key Points
-
Glycosylation is a key cellular mechanism regulating many important biological processes in cancer; alterations in glycosylation patterns are common features of cancer cells
-
The prostate is a secretory gland with an important role in male fertility, and as such, is an abundant secretor of glycoproteins of all types
-
A variety of alterations in glycoproteins have been observed in prostate cancer cells including: increased sialylation and fucosylation; increased O-β-N-acetylglucosylation; the emergence of cryptic and high-mannose N-glycans and proteoglycan alterations
-
Glycosylation-specific antibodies and glycosylation gene signatures have proven to be powerful tools in classifying prostate cancer tumour aggressiveness
-
Glycans have potential clinical applications in patients with prostate cancer as both predictive and prognostic biomarkers and therapeutic targets
Abstract
Prostate cancer is a unique and heterogeneous disease. Currently, a major unmet clinical need exists to develop biomarkers that enable indolent disease to be distinguished from aggressive disease. The prostate is an abundant secretor of glycoproteins of all types, and alterations in glycans are, therefore, attractive as potential biomarkers and therapeutic targets. Despite progress over the past decade in profiling the genome and proteome, the prostate cancer glycoproteome remains relatively understudied. A wide range of alterations in the glycoproteins on prostate cancer cells can occur, including increased sialylation and fucosylation, increased O-β-N-acetylglucosamine (GlcNAc) conjugation, the emergence of cryptic and high-mannose N-glycans and alterations to proteoglycans. Glycosylation can alter protein function and has a key role in many important biological processes in cancer including cell adhesion, migration, interactions with the cell matrix, immune surveillance, cell signalling and cellular metabolism; altered glycosylation in prostate cancer might modify some, or all of these processes. In the past three years, powerful tools such as glycosylation-specific antibodies and glycosylation gene signatures have been developed, which enable detailed analyses of changes in glycosylation. Thus, emerging data on these often overlooked modifications have the potential to improve risk stratification and therapeutic strategies in patients with prostate cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Klotz, L. & Emberton, M. Management of low risk prostate cancer-active surveillance and focal therapy. Nat. Rev. Clin. Oncol. 11, 324–334 (2014).
Humphrey, P. A. Gleason grading and prognostic factors in carcinoma of the prostate. Mod. Pathol. 17, 292–306 (2004).
Massie, C. E. et al. The androgen receptor fuels prostate cancer by regulating central metabolism and biosynthesis. EMBO J. 30, 2719–2733 (2011).
Sharma, N. L. et al. The androgen receptor induces a distinct transcriptional program in castration-resistant prostate cancer in man. Cancer Cell 23, 35–47 (2013).
Mills, I. G. Maintaining and reprogramming genomic androgen receptor activity in prostate cancer. Nat. Rev. Cancer 14, 187–198 (2014).
Stray-Pedersen, A. et al. PGM3 mutations cause a congenital disorder of glycosylation with severe immunodeficiency and skeletal dysplasia. Am. J. Hum. Genet. 95, 96–107 (2014).
Hakomori, S. I. & Murakami, W. T. Glycolipids of hamster fibroblasts and derived malignant-transformed cell lines. Proc. Natl Acad. Sci. USA 59, 254–261 (1968).
Ladenson, R. P., Schwartz, S. O. & Ivy, A. C. Incidence of the blood groups and the secretor factor in patients with pernicious anemia and stomach carcinoma. Am. J. Med. Sci. 217, 194–197 (1949).
Hakomori, S. & Kannagi, R. Glycosphingolipids as tumor-associated and differentiation markers. J. Natl Cancer Inst. 71, 231–251 (1983).
Johnson, V. G. et al. Analysis of a human tumor-associated glycoprotein (TAG-72) identified by monoclonal antibody B72.3. Cancer Res. 46, 850–857 (1986).
Herlyn, M., Sears, H. F., Steplewski, Z. & Koprowski, H. Monoclonal antibody detection of a circulating tumor-associated antigen. I. Presence of antigen in sera of patients with colorectal, gastric, and pancreatic carcinoma. J. Clin. Immunol. 2, 135–140 (1982).
Klug, T. L. et al. Monoclonal antibody immunoradiometric assay for an antigenic determinant (CA 125) associated with human epithelial ovarian carcinomas. Cancer Res. 44, 1048–1053 (1984).
Munkley, J. & Elliott D. J. Hallmarks of glycosylation in cancer. Oncotarget http://dx.doi.org/10.18632/oncotarget.8155 (2016)
Lilja, H., Ulmert, D. & Vickers, A. J. Prostate-specific antigen and prostate cancer: prediction, detection and monitoring. Nat. Rev. Cancer 8, 268–278 (2008).
Dhanasekaran, S. M. et al. Delineation of prognostic biomarkers in prostate cancer. Nature 412, 822–826 (2001).
Barry, M. J. Prostate-specific-antigen testing for early diagnosis of prostate cancer. N. Engl. J. Med. 344, 1373–1377 (2001).
Barry, M. J. Screening for prostate cancer — the controversy that refuses to die. N. Engl. J. Med. 360, 1351–1354 (2009).
Gilgunn, S., Conroy, P. J., Saldova, R., Rudd, P. M. & O'Kennedy, R. J. Aberrant PSA glycosylation — a sweet predictor of prostate cancer. Nat. Rev. Urol. 10, 99–107 (2013). Review article discussing the potential of altered PSA-glycosylation patterns to be used diagnostically to distinguish indolent from aggressive prostate cancer.
Drake, R. R., Jones, E., E., Powers, T. W. & Nyalwidhe, J. O. Chapter ten — altered glycosylation in prostate cancer. Adv. Cancer Res. 126, 345–382 (2015).
Okada, T. et al. Structural characteristics of the N-glycans of two isoforms of prostate-specific antigens purified from human seminal fluid. Biochim. Biophys. Acta 1525, 149–160 (2001).
Prakash, S. & Robbins, P. W. Glycotyping of prostate specific antigen. Glycobiology 10, 173–176 (2000).
Peracaula, R. et al. Altered glycosylation pattern allows the distinction between prostate-specific antigen (PSA) from normal and tumor origins. Glycobiology 13, 457–470 (2003).
White, K. Y. et al. Glycomic characterization of prostate-specific antigen and prostatic acid phosphatase in prostate cancer and benign disease seminal plasma fluids. J. Proteome Res. 8, 620–630 (2009).
Tajiri, M., Ohyama, C. & Wada, Y. Oligosaccharide profiles of the prostate specific antigen in free and complexed forms from the prostate cancer patient serum and in seminal plasma: a glycopeptide approach. Glycobiology 18, 2–8 (2008).
Dwek, M. V., Jenks, A. & Leathem, A. J. A sensitive assay to measure biomarker glycosylation demonstrates increased fucosylation of prostate specific antigen (PSA) in patients with prostate cancer compared with benign prostatic hyperplasia. Clin. Chim. Acta 411, 1935–1939 (2010).
Fukushima, K., Satoh, T., Baba, S. & Yamashita, K. α1,2-fucosylated and β-N-acetylgalactosaminylated prostate-specific antigen as an efficient marker of prostatic cancer. Glycobiology 20, 452–460 (2010).
Sarrats, A. et al. Differential percentage of serum prostate-specific antigen subforms suggests a new way to improve prostate cancer diagnosis. Prostate 70, 1–9 (2010).
Sarrats, A. et al. Glycan characterization of PSA 2-DE subforms from serum and seminal plasma. OMICS 14, 465–474 (2010).
Ohyama, C. et al. Carbohydrate structure and differential binding of prostate specific antigen to Maackia amurensis lectin between prostate cancer and benign prostate hypertrophy. Glycobiology 14, 671–679 (2004).
Meany, D. L. & Chan, D. W. Aberrant glycosylation associated with enzymes as cancer biomarkers. Clin. Proteomics 8, 7 (2011).
Chen, Z., Gulzar, Z. G., St Hill, C. A., Walcheck, B. & Brooks, J. D. Increased expression of GCNT1 is associated with altered O-glycosylation of PSA, PAP, and MUC1 in human prostate cancers. Prostate 74, 1059–1067 (2014). Core-2-GlcNAc-transferase (GCNT1) is over-expressed in prostate cancer and is associated with higher levels of core-2-O-sLe(x) in PSA, PAP and MUC1 proteins. Alterations of O-linked glycosylation could be important in prostate cancer biology and could provide a new avenue for development of prostate cancer specific glycoprotein biomarkers.
Leymarie, N. et al. Interlaboratory study on differential analysis of protein glycosylation by mass spectrometry: the ABRF glycoprotein research multi-institutional study 2012. Mol. Cell. Proteomics 12, 2935–2951 (2013).
Stura, E. A. et al. Crystal structure of human prostate-specific antigen in a sandwich antibody complex. J. Mol. Biol. 414, 530–544 (2011).
Vanhooren, V. et al. Serum N-glycan profile shift during human ageing. Exp. Gerontol. 45, 738–743 (2010).
Yoshida, K. I. et al. Serial lectin affinity chromatography with concavalin A and wheat germ agglutinin demonstrates altered asparagine-linked sugar-chain structures of prostatic acid phosphatase in human prostate carcinoma. J. Chromatogr. B Biomed. Sci. Appl. 695, 439–443 (1997).
Kudelka, M. R., Ju, T., Heimburg-Molinaro, J. & Cummings, R. D. Chapter three — imple sugars to complex disease — mucin-type O-glycans in cancer. Adv. Cancer Res. 126, 53–135 (2015).
Pinho, S. S. & Reis, C. A. Glycosylation in cancer: mechanisms and clinical implications. Nat. Rev. Cancer 15, 540–555 (2015).
Radhakrishnan, P. et al. Immature truncated O-glycophenotype of cancer directly induces oncogenic features. Proc. Natl Acad. Sci. USA 111, E4066–E4075 (2014).
Kobayashi, H., Terao, T. & Kawashima, Y. Sialyl Tn as a prognostic marker in epithelial ovarian cancer. Br. J. Cancer 66, 984–985 (1992).
Kobayashi, H., Terao, T. & Kawashima, Y. Serum sialyl Tn as an independent predictor of poor prognosis in patients with epithelial ovarian cancer. J. Clin. Oncol. 10, 95–101 (1992).
Soares, R., Marinho, A. & Schmitt, F. Expression of sialyl-Tn in breast cancer correlation with prognostic parameters. Pathol. Res. Pract. 192, 1181–1186 (1996).
Munkley, J. The role of Sialyl-Tn in cancer. Int. J. Mol. Sci. 17, 275 (2016).
Arai, T., Fujita, K., Fujime, M. & Irimura, T. Expression of sialylated MUC1 in prostate cancer: relationship to clinical stage and prognosis. Int. J. Urol. 12, 654–661 (2005).
Munkley, J. et al. The androgen receptor controls expression of the cancer-associated sTn antigen and cell adhesion through induction of ST6GalNAc1 in prostate cancer. Oncotarget 6, 34358–34374 (2015). The sialytransferase ST6GalNAc1 and the cancer-associated sialyl-Tn antigen are regulated by androgens in prostate cancer cells. Surprisingly, given its high expression in tumours, stable expression of ST6GalNAc1 in prostate cancer cells reduced the formation of stable tumours in mice, reduced cell adhesion and induced a switch towards a more mesenchymal-like cell phenotype in vitro. ST6GalNAc1 has a dynamic expression pattern in clinical datasets, being significantly up-regulated in primary prostate carcinoma but relatively down-regulated in established metastatic tissue.
Genega, E. M., Hutchinson, B., Reuter, V. E. & Gaudin, P. B. Immunophenotype of high-grade prostatic adenocarcinoma and urothelial carcinoma. Mod. Pathol. 13, 1186–1191 (2000).
Munkley, J. The role of sialyl-Tn in cancer. Int. J. Mol. Sci. 17, E275 (2016).
Myers, R. B. et al. Tumor associated glycoprotein-72 is highly expressed in prostatic adenocarcinomas. J. Urol. 152, 243–246 (1994).
Ozaki, H. et al. Enhancement of metastatic ability by ectopic expression of ST6GalNAcI on a gastric cancer cell line in a mouse model. Clin. Exp. Metastasis 29, 229–238 (2012).
Julien, S. et al. ST6GalNAc I expression in MDA-MB-231 breast cancer cells greatly modifies their O-glycosylation pattern and enhances their tumourigenicity. Glycobiology 16, 54–64 (2006).
Slovin, S. F. et al. Fully synthetic carbohydrate-based vaccines in biochemically relapsed prostate cancer: clinical trial results with α-N-acetylgalactosamine-O-serine/threonine conjugate vaccine. J. Clin. Oncol. 21, 4292–4298 (2003).
Amado, M., Carneiro, F., Seixas, M., Clausen, H. & Sobrinho-Simoes, M. Dimeric sialyl-Lex expression in gastric carcinoma correlates with venous invasion and poor outcome. Gastroenterology 114, 462–470 (1998).
Baldus, S. E. et al. Histopathological subtypes and prognosis of gastric cancer are correlated with the expression of mucin-associated sialylated antigens: Sialosyl-Lewisa, Sialosyl-Lewisx and sialosyl-Tn. Tumour Biol. 19, 445–453 (1998).
Walker, P. D., Karnik, S., deKernion, J. B. & Pramberg, J. C. Cell surface blood group antigens in prostatic carcinoma. Am. J. Clin. Pathol. 81, 503–506 (1984).
Culig, Z. et al. Expression of Lewis carbohydrate antigens in metastatic lesions from human prostatic carcinoma. Prostate 36, 162–167 (1998).
Jorgensen, T. et al. Up-regulation of the oligosaccharide sialyl Lewisx: a new prognostic parameter in metastatic prostate cancer. Cancer Res. 55, 1817–1819 (1995).
Martensson, S. et al. Sialyl-Lewisx and related carbohydrate antigens in the prostate. Hum. Pathol. 26, 735–739 (1995).
Okamoto, T. et al. Core2 O-glycan-expressing prostate cancer cells are resistant to NK cell immunity. Mol. Med. Rep. 7, 359–364 (2013).
Carvalho, A. S. et al. Differential expression of α-2,3-sialyltransferases and α-1,3/4-fucosyltransferases regulates the levels of sialyl Lewis a and sialyl Lewis x in gastrointestinal carcinoma cells. Int. J. Biochem. Cell Biol. 42, 80–89 (2010).
Saldova, R., Fan, Y., Fitzpatrick, J. M., Watson, R. W. & Rudd, P. M. Core fucosylation and α2-3 sialylation in serum N-glycome is significantly increased in prostate cancer comparing to benign prostate hyperplasia. Glycobiology 21, 195–205 (2011).
Tabares, G. et al. Different glycan structures in prostate-specific antigen from prostate cancer sera in relation to seminal plasma PSA. Glycobiology 16, 132–145 (2006).
Kyselova, Z. et al. Alterations in the serum glycome due to metastatic prostate cancer. J. Proteome Res. 6, 1822–1832 (2007).
Shah, P. et al. Integrated proteomic and glycoproteomic analyses of prostate cancer cells reveal glycoprotein alteration in protein abundance and glycosylation. Mol. Cell. Proteomics 14, 2753–2763 (2015).
Wang, X. et al. Overexpression of α (1,6) fucosyltransferase associated with aggressive prostate cancer. Glycobiology 24, 935–944 (2014).
Barthel, S. R. et al. Alpha 1,3 fucosyltransferases are master regulators of prostate cancer cell trafficking. Proc. Natl Acad. Sci. USA 106, 19491–19496 (2009).
Lange, T. et al. Human prostate cancer in a clinically relevant xenograft mouse model: identification of β(1,6)-branched oligosaccharides as a marker of tumor progression. Clin. Cancer Res. 18, 1364–1373 (2012).
Tsui, K. H. et al. Evaluating the function of matriptase and N-acetylglucosaminyltransferase V in prostate cancer metastasis. Anticancer Res. 28, 1993–1999 (2008).
Ishibashi, Y. et al. Serum tri- and tetra-antennary N-glycan is a potential predictive biomarker for castration-resistant prostate cancer. Prostate 74, 1521–1529 (2014).
Newsom-Davis, T. E. et al. Enhanced immune recognition of cryptic glycan markers in human tumors. Cancer Res. 69, 2018–2025 (2009).
Wang, D. et al. N-glycan cryptic antigens as active immunological targets in prostate cancer patients. J. Proteomics Bioinform. 5, 090–095 (2012).
Wang, D. et al. Anti-oligomannose antibodies as potential serum biomarkers of aggressive prostate cancer. Drug Dev. Res. 74, 65–80 (2013).
Ravindranath, M. H. et al. Gangliosides of organ-confined versus metastatic androgen-receptor-negative prostate cancer. Biochem. Biophys. Res. Commun. 324, 154–165 (2004).
Hatano, K. et al. Androgen-regulated transcriptional control of sialyltransferases in prostate cancer cells. PLoS ONE 7, e31234 (2012).
Nonaka, M. et al. Determination of carbohydrate structure recognized by prostate-specific F77 monoclonal antibody through expression analysis of glycosyltransferase genes. J. Biol. Chem. 289, 16478–16486 (2014). The F77 antibody was initially isolated from mice after injection of PC3 tumour cells and provoked great interest based on its diagnostic and therapeutic potential. The glycosyltransferases responsible for the synthesis of the F77 antigen have been identified as FUT1, GCNT1, GCNT2 and GCNT3. Notably the core-2-glycosyltransferase GCNT1 is upregulated in prostate cancer tissue and this upregulation has been previously linked with disease progression.
Gao, C. et al. Carbohydrate sequence of the prostate cancer-associated antigen F77 assigned by a mucin O-glycome designer array. J. Biol. Chem. 289, 16462–16477 (2014).
Carroll, A. M. et al. Monoclonal antibodies to tissue-specific cell surface antigens. I. Characterization of an antibody to a prostate tissue antigen. Clin. Immunol. Immunopathol. 33, 268–281 (1984).
Zhang, G. et al. Suppression of human prostate tumor growth by a unique prostate-specific monoclonal antibody F77 targeting a glycolipid marker. Proc. Natl Acad. Sci. USA 107, 732–737 (2010).
Hagisawa, S. et al. Expression of core 2 β1,6-N-acetylglucosaminyltransferase facilitates prostate cancer progression. Glycobiology 15, 1016–1024 (2005).
Ricciardelli, C. et al. Elevated stromal chondroitin sulfate glycosaminoglycan predicts progression in early-stage prostate cancer. Clin. Cancer Res. 3, 983–992 (1997).
Iozzo, R. V. & Sanderson, R. D. Proteoglycans in cancer biology, tumour microenvironment and angiogenesis. J. Cell. Mol. Med. 15, 1013–1031 (2011).
Afratis, N. et al. Glycosaminoglycans: key players in cancer cell biology and treatment. FEBS J. 279, 1177–1197 (2012).
Edwards, I. J. Proteoglycans in prostate cancer. Nat. Rev. Urol. 9, 196–206 (2012).
Suhovskih, A. V. et al. Proteoglycan expression in normal human prostate tissue and prostate cancer. ISRN Oncol. 2013, 680136 (2013).
Henke, A. et al. Stromal expression of decorin, Semaphorin6D, SPARC, Sprouty1 and Tsukushi in developing prostate and decreased levels of decorin in prostate cancer. PLoS ONE 7, e42516 (2012).
Hu, Y. et al. Decorin suppresses prostate tumor growth through inhibition of epidermal growth factor and androgen receptor pathways. Neoplasia 11, 1042–1053 (2009).
Datta, M. W. et al. Perlecan, a candidate gene for the CAPB locus, regulates prostate cancer cell growth via the Sonic Hedgehog pathway. Mol. Cancer 5, 9 (2006).
Ricciardelli, C. et al. Elevated levels of versican but not decorin predict disease progression in early-stage prostate cancer. Clin. Cancer Res. 4, 963–971 (1998).
Touab, M., Villena, J., Barranco, C., Arumi-Uria, M. & Bassols, A. Versican is differentially expressed in human melanoma and may play a role in tumor development. Am. J. Pathol. 160, 549–557 (2002).
Pukkila, M. et al. High stromal versican expression predicts unfavourable outcome in oral squamous cell carcinoma. J. Clin. Pathol. 60, 267–272 (2007).
Skandalis, S. S., Kletsas, D., Kyriakopoulou, D., Stavropoulos, M. & Theocharis, D. A. The greatly increased amounts of accumulated versican and decorin with specific post-translational modifications may be closely associated with the malignant phenotype of pancreatic cancer. Biochim. Biophys. Acta 1760, 1217–1225 (2006).
Sakko, A. J. et al. Immunohistochemical level of unsulfated chondroitin disaccharides in the cancer stroma is an independent predictor of prostate cancer relapse. Cancer Epidemiol. Biomarkers Prev. 17, 2488–2497 (2008).
Ricciardelli, C. et al. Formation of hyaluronan- and versican-rich pericellular matrix by prostate cancer cells promotes cell motility. 282, 10814–10825 (2007).
Ricciardelli, C. et al. Elevated levels of peritumoral chondroitin sulfate are predictive of poor prognosis in patients treated by radical prostatectomy for early-stage prostate cancer. Cancer Res. 59, 2324–2328 (1999).
Shimada, K. et al. Syndecan-1 (CD138) contributes to prostate cancer progression by stabilizing tumour-initiating cells. J. Pathol. 231, 495–504 (2013).
Fujii, T. et al. Syndecan-1 up-regulates microRNA-331-3p and mediates epithelial-to-mesenchymal transition in prostate cancer. Mol. Carcinog. http://dx.doi.org/10.1002/mc.22381 (2015).
Fujii, T., Shimada, K., Tatsumi, Y., Fujimoto, K. & Konishi, N. Syndecan-1 responsive microRNA-126 and 149 regulate cell proliferation in prostate cancer. Biochem. Biophys. Res. Commun. 456, 183–189 (2015).
Love, D. C. & Hanover, J. A. The hexosamine signaling pathway: deciphering the 'O-GlcNAc code'. Sci. signal. 2005, re13 (2005).
Hanover, J. A., Krause, M. W. & Love, D. C. Bittersweet memories: linking metabolism to epigenetics through O-GlcNAcylation. Nat. Rev. Mol. Cell Biol. 13, 312–321 (2012).
Bond, M. R. & Hanover, J. A. A little sugar goes a long way: the cell biology of O-GlcNAc. J. Cell Biol. 208, 869–880 (2015).
Butkinaree, C., Park, K. & Hart, G. W. O-linked β-N-acetylglucosamine (O-GlcNAc): extensive crosstalk with phosphorylation to regulate signaling and transcription in response to nutrients and stress. Biochim. Biophys. Acta 1800, 96–106 (2010).
Schwarz, F. & Aebi, M. Mechanisms and principles of N-linked protein glycosylation. Curr. Opin. Struct. Biol. 21, 576–582 (2011).
Itkonen, H. M. et al. O-GlcNAc transferase integrates metabolic pathways to regulate the stability of c-MYC in human prostate cancer cells. Cancer Res. 73, 5277–5287 (2013).
Lynch, T. P. et al. Critical role of O-linked β-N-acetylglucosamine transferase in prostate cancer invasion, angiogenesis, and metastasis. J. Biol. Chem. 287, 11070–11081 (2012).
Kamigaito, T. et al. Overexpression of O-GlcNAc by prostate cancer cells is significantly associated with poor prognosis of patients. Prostate Cancer Prostatic Dis. 17, 18–22 (2014).
Itknonen, H.M. et al. Inhibition of O-GlcNAc transferase activity reprograms prostate cancer cell metabolism. http://dx.doi.org/10.18632/oncotarget.7039 (2016).
Itkonen, H. M. et al. UAP1 is overexpressed in prostate cancer and is protective against inhibitors of N-linked glycosylation. Oncogene 34, 3744–3750 (2014). The hexosamine biosynthetic pathway (HBP) produces hexosamines that are used by enzymes in the endoplasmic reticulum and golgi apparatus to glycosylate proteins targeted to the plasma membrane and those that are secreted. This study demonstrates that the HBP enzyme UAP1, which is regulated by androgens in prostate cancer cells, is linked with cancer progression. High levels of UAP1 expression and/or HBP activity appear to be protective against inhibitors of N-linked glycosylation in prostate cancer.
Barfeld, S. J., East, P., Zuber, V. & Mills, I. G. Meta-analysis of prostate cancer gene expression data identifies a novel discriminatory signature enriched for glycosylating enzymes. BMC Med. Genom. 7, 513 (2014). This study identifies a 33-gene signature that can discriminate between benign tissue controls and localised prostate cancers irrespective of detection platform or dissection status. Biologically, glycosylation was the single enriched process associated with this 33 gene signature, encompassing four glycosylating enzymes.
Itkonen, H. M. & Mills, I. G. N-linked glycosylation supports cross-talk between receptor tyrosine kinases and androgen receptor. PLoS ONE 8, e65016 (2013). This study shows that glycosylation of the insulin-like growth factor-1 (IGFR1) is necessary for the full activation of the receptor in response to androgen treatment and that perturbing this process can break the feedback loop between AR and IGFR1 activation in prostate cells.
Mitchell, B. S. & Schumacher, U. The use of the lectin Helix pomatia agglutinin (HPA) as a prognostic indicator and as a tool in cancer research. Histol. Histopathol. 14, 217–226 (1999).
Laack, E. et al. Lectin histochemistry of resected adenocarcinoma of the lung: helix pomatia agglutinin binding is an independent prognostic factor. Am. J. Pathol. 160, 1001–1008 (2002).
Kakeji, Y., Tsujitani, S., Mori, M., Maehara, Y. & Sugimachi, K. Helix pomatia agglutinin binding activity is a predictor of survival time for patients with gastric carcinoma. Cancer 68, 2438–2442 (1991).
Thies, A., Moll, I., Berger, J. & Schumacher, U. Lectin binding to cutaneous malignant melanoma: HPA is associated with metastasis formation. Br. J. Cancer 84, 819–823 (2001).
Lange, T. et al. Aberrant presentation of HPA-reactive carbohydrates implies selectin-independent metastasis formation in human prostate cancer. Clin. Cancer Res. 20, 1791–1802 (2014). Positive binding of the Helix pomatia (HPA) lectin is associated with disease progression and metastasis in a range of cancer types; however, in prostate cancer, binding of HPA is reduced in metastatic disease and patients with HPA-negative tumours have worse overall survival and faster progression.
Powers, T. W. et al. Matrix assisted laser desorption ionization imaging mass spectrometry workflow for spatial profiling analysis of N-linked glycan expression in tissues. Anal. Chem. 85, 9799–9806 (2013).
Powers, T. W. et al. MALDI imaging mass spectrometry profiling of N-glycans in formalin-fixed paraffin embedded clinical tissue blocks and tissue microarrays. PLoS ONE 9, e106255 (2014).
Yoneyama, T. et al. Measurement of aberrant glycosylation of prostate specific antigen can improve specificity in early detection of prostate cancer. Biochem. Biophys. Res. Commun. 448, 390–396 (2014).
Drake, R. R. & Kislinger, T. The proteomics of prostate cancer exosomes. Expert Rev. Proteomics 11, 167–177 (2014).
Taniguchi, N. & Kizuka, Y. Chapter two — glycans and cancer: role of N-glycans in cancer biomarker, progression and metastasis, and therapeutics. Adv. Cancer Res. 126, 11–51 (2015).
Rini, J., Esko, J. & Varki, A. et al. Essentials of Glycobiology (eds Varki, A. et al.) (2009).
Acknowledgements
This work was funded by Prostate Cancer UK (PG12-34), The J.G.W Patterson Foundation, The Wellcome Trust (grant numbers WT080368MA and WT089225/Z/09/Z) and BBSRC (grant BB/1006923/1 and BB/J007293/1). I.G.M is supported in Belfast by the Belfast-Manchester Movember Centre of Excellence (CE013_2-004), funded in partnership with Prostate Cancer UK.
Author information
Authors and Affiliations
Contributions
J.M. researched data for this article, J.M. and D.E. made a substantial contribution to discussions of content, J.M. and I.G.M. wrote the manuscript and all authors edited and/or reviewed the manuscript prior to submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Glycoproteins
-
Proteins modified by one or more glycans.
- Glycans
-
The carbohydrate moieties of a glycoprotein, glycolipid or a proteoglycan.
- O-linked glycans
-
Oligosaccharides that are covalently linked to a serine or threonine residue via an oxygen atom.
- N-linked glycans
-
Oligosaccharides covalently linked to an asparagine residue of a protein via a nitrogen atom.
- Glycome
-
The global collection of glycans (sugar chains) synthesized by a cell.
- Glycosyltransferases
-
Enzymes that catalyse the transfer of sugars (saccharides) to proteins, lipids or carbohydrates, forming covalent bonds.
- Lectins
-
Carbohydrate binding proteins that are highly specific for sugar moieties.
- Glycolipid
-
A lipid modified by one or more glycans.
- Proteoglycans
-
Proteins with one or more glycosaminoglycan (GAG) chains, such as chondroitin sulfate and heparin sulfate. Proteoglycans constitute a large fraction of the extracellular matrix and have key roles in cell adhesion and motility.
- O-GlcNAcylation
-
Addition of N-acetylglucosamine (GlcNAc) to serine or threonine residues on nuclear and cytoplasmic proteins.
Rights and permissions
About this article
Cite this article
Munkley, J., Mills, I. & Elliott, D. The role of glycans in the development and progression of prostate cancer. Nat Rev Urol 13, 324–333 (2016). https://doi.org/10.1038/nrurol.2016.65
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrurol.2016.65
This article is cited by
-
The role of GCNT1 mediated O-glycosylation in aggressive prostate cancer
Scientific Reports (2023)
-
Upregulation of GALNT7 in prostate cancer modifies O-glycosylation and promotes tumour growth
Oncogene (2023)
-
An N-glycoproteomic site-mapping analysis reveals glycoprotein alterations in esophageal squamous cell carcinoma
Journal of Translational Medicine (2022)
-
Simultaneous analysis of serum α2,3-linked sialylation and core-type fucosylation of prostate-specific antigen for the detection of high-grade prostate cancer
British Journal of Cancer (2022)
-
Systems glycobiology for discovering drug targets, biomarkers, and rational designs for glyco-immunotherapy
Journal of Biomedical Science (2021)