Key Points
-
Genomic signatures, molecular imaging techniques and the presence or absence of disseminated and circulating tumour cells can identify patients harbouring occult metastases who can benefit from combined therapies
-
In high-risk and/or locally advanced prostate cancer, the combination of image-guided radiotherapy (IGRT) and androgen deprivation therapy (ADT) improves overall survival compared with monotherapies
-
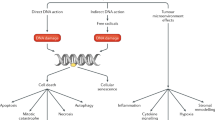
ADT with IGRT can improve outcomes by eradicating occult systemic metastases, improving local and systemic control and decreasing the ability of prostate cancer cells to repair IGRT-induced DNA damage
-
No biomarkers are currently in clinical use to help decide which patients will benefit from ADT
-
Biomarkers of occult metastatic disease and of the efficacy of ADT in individual patients would enable a more bespoke approach to the use, duration and timing of ADT–IGRT
Abstract
The combined use of androgen deprivation therapy (ADT) and image-guided radiotherapy (IGRT) can improve overall survival in aggressive, localized prostate cancer. However, owing to the adverse effects of prolonged ADT, it is imperative to identify the patients who would benefit from this combined-modality therapy relative to the use of IGRT alone. Opportunities exist for more personalized approaches in treating aggressive, locally advanced prostate cancer. Biomarkers—such as disseminated tumour cells, circulating tumour cells, genomic signatures and molecular imaging techniques—could identify the patients who are at greatest risk for systemic metastases and who would benefit from the addition of systemic ADT. By contrast, when biomarkers of systemic disease are not present, treatment could proceed using local IGRT alone. The choice of drug, treatment duration and timing of ADT relative to IGRT could be predicated on these personalized approaches to prostate cancer medicine. These novel treatment intensification and reduction strategies could result in improved prostate-cancer-specific survival and overall survival, without incurring the added expense of metabolic syndrome and other adverse effects of ADT in all patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
American Cancer Society. What are the key statistics about prostate cancer? [online], (2014).
International Agency for Cancer Research. Prostate cancer: estimated incidence, mortality & prevalence, 2012 [online], (2014).
Mohler, J. et al. NCCN clinical practice guidelines in oncology: prostate cancer. J. Natl Compr. Canc. Netw. 8, 162–200 (2010).
Nichol, A. M., Warde, P. & Bristow, R. G. Optimal treatment of intermediate-risk prostate carcinoma with radiotherapy: clinical and translational issues. Cancer 104, 891–905 (2005).
D'Amico, A. V. et al. Optimizing patient selection for dose escalation techniques using the prostate-specific antigen level, biopsy gleason score, and clinical T-stage. Int. J. Radiat. Oncol. Biol. Phys. 45, 1227–1233 (1999).
Hernandez, D. J., Nielsen, M. E., Han, M. & Partin, A. W. Contemporary evaluation of the D'amico risk classification of prostate cancer. Urology 70, 931–935 (2007).
Montironi, R. et al. Consensus statement with recommendations on active surveillance inclusion criteria and definition of progression in men with localized prostate cancer: the critical role of the pathologist. Virchows Arch. 465, 623–628 (2014).
Magi-Galluzzi, C. et al. International Society of Urological Pathology (ISUP) Consensus Conference on Handling and Staging of Radical Prostatectomy Specimens. Working group 3: extraprostatic extension, lymphovascular invasion and locally advanced disease. Mod. Pathol. 24, 26–38 (2011).
Zelefsky, M. J. et al. Improved clinical outcomes with high-dose image guided radiotherapy compared with non-IGRT for the treatment of clinically localized prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 84, 125–129 (2012).
Heemsbergen, W. D. et al. Increased risk of biochemical and clinical failure for prostate patients with a large rectum at radiotherapy planning: results from the Dutch trial of 68 Gy versus 78 Gy. Int. J. Radiat. Oncol. Biol. Phys. 67, 1418–1424 (2007).
de Crevoisier, R. et al. Increased risk of biochemical and local failure in patients with distended rectum on the planning CT for prostate cancer radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 62, 965–973 (2005).
Mendenhall, N. P. et al. Five-year outcomes from 3 prospective trials of image-guided proton therapy for prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 88, 596–602 (2014).
Zaorsky, N. G. et al. Evolution of advanced technologies in prostate cancer radiotherapy. Nat. Rev. Urol. 10, 565–579 (2013).
Morgan, P. B. et al. Timing of biochemical failure and distant metastatic disease for low-, intermediate-, and high-risk prostate cancer after radiotherapy. Cancer 110, 68–80 (2007).
D'Amico, A. V., Cote, K., Loffredo, M., Renshaw, A. A. & Schultz, D. Determinants of prostate cancer specific survival following radiation therapy during the prostate specific antigen era. J. Urol. 170, S42–S47 (2003).
Gilbert, S. M., Kuo, Y. F. & Shahinian, V. B. Prevalent and incident use of androgen deprivation therapy among men with prostate cancer in the United States. Urol. Oncol. 29, 647–653 (2011).
Berg, A. et al. Impact of disseminated tumor cells in bone marrow at diagnosis in patients with nonmetastatic prostate cancer treated by definitive radiotherapy. Int. J. Cancer 120, 1603–1609 (2007).
Attard, G. et al. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J. Clin. Oncol. 27, 3742–3748 (2009).
Scher, H. I. et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1–2 study. Lancet 375, 1437–1446 (2010).
Armstrong, A. J. Biomarkers in castration-resistant prostate cancer. Clin. Adv. Hematol. Oncol. 12, 115–118 (2014).
Kohli, M., Qin, R., Jimenez, R. & Dehm, S. M. Biomarker-based targeting of the androgen-androgen receptor axis in advanced prostate cancer. Adv. Urol. http://dx.doi.org/10.1155/2012/781459 (2012).
Carducci, M. Intermediate Clinical Endpoint of Prostate Cancer (ICECaP) Effort. ASCO University [online], (2013).
Buyyounouski, M. K., Pickles, T., Kestin, L. L., Allison, R. & Williams, S. G. Validating the interval to biochemical failure for the identification of potentially lethal prostate cancer. J. Clin. Oncol. 30, 1857–1863 (2012).
Bolla, M. et al. Duration of androgen suppression in the treatment of prostate cancer. N. Engl. J. Med. 360, 2516–2527 (2009).
Souhami, L., Bae, K., Pilepich, M. & Sandler, H. Impact of the duration of adjuvant hormonal therapy in patients with locally advanced prostate cancer treated with radiotherapy: a secondary analysis of RTOG 85–31 J. Clin. Oncol. 27, 2137–2143 (2009).
Pilepich, M. V. et al. Phase III radiation therapy oncology group (RTOG) trial 86–10 of androgen deprivation adjuvant to definitive radiotherapy in locally advanced carcinoma of the prostate. Int. J. Radiat. Oncol. Biol. Phys. 50, 1243–1252 (2001).
Warde, P. et al. Combined androgen deprivation therapy and radiation therapy for locally advanced prostate cancer: a randomised, phase 3 trial. Lancet 378, 2104–2111 (2011).
Bolla, M. et al. External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk: 10-year results of an EORTC randomised study. Lancet Oncol. 11, 1066–1073 (2010).
Michalski, J. et al. Clinical outcome of patients treated with 3D conformal radiation therapy (3D-CRT) for prostate cancer on RTOG 9406. Int. J. Radiat. Oncol. Biol. Phys. 83, e363–70 (2012).
Zelefsky, M. J. et al. Dose escalation for prostate cancer radiotherapy: predictors of long-term biochemical tumor control and distant metastases-free survival outcomes. Eur. Urol. 60, 1133–1139 (2011).
Zumsteg, Z. S. et al. Short-term androgen-deprivation therapy improves prostate cancer-specific mortality in intermediate-risk prostate cancer patients undergoing dose-escalated external beam radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 85, 1012–1017 (2013).
Zelefsky, M. J., Reuter, V. E., Fuks, Z., Scardino, P. & Shippy, A. Influence of local tumor control on distant metastases and cancer related mortality after external beam radiotherapy for prostate cancer. J. Urol. 179, 1368–1373 (2008).
Zapatero, A. et al. Long-term versus short-term androgen deprivation combined with high-dose radiotherapy for intermediate and high-risk prostate cancer: A randomized controlled trial (DART01/05) [abstract 5038]. J. Clin. Oncol. 32 (Suppl.), 5s (2014).
Dearnaley, D. P. et al. Escalated-dose versus standard-dose conformal radiotherapy in prostate cancer: first results from the MRC RT01 randomised controlled trial. Lancet Oncol. 8, 475–487 (2007).
Hennequin, C. Radiation Therapy in Treating Patients Receiving Hormone Therapy for Prostate Cancer. Clinicaltrials.gov [online], (2009).
Nabid, A. Study on the Role of Hormonal Treatment for Two Dosage Levels of Prostate Radiation Therapy Versus Prostate Radiation Therapy Alone (PCS III). Clinicaltrials.gov [online], (2015).
Bolla, M. Radiation Therapy With or Without Bicalutamide and Goserelin in Treating Patients With Prostate Cancer. Clinicaltrials.gov [online], (2011).
Jereczek-Fossa, B. A. et al. Robotic image-guided stereotactic radiotherapy, for isolated recurrent primary, lymph node or metastatic prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 82, 889–897 (2012).
Singh, D. et al. Is there a favorable subset of patients with prostate cancer who develop oligometastases? Int. J. Radiat. Oncol. Biol. Phys. 58, 3–10 (2004).
Corbin, K. S., Hellman, S. & Weichselbaum, R. R. Extracranial oligometastases: a subset of metastases curable with stereotactic radiotherapy. J. Clin. Oncol. 31, 1384–1390 (2013).
Berkovic, P. et al. Salvage stereotactic body radiotherapy for patients with limited prostate cancer metastases: deferring androgen deprivation therapy. Clin. Genitourin. Cancer 11, 27–32 (2013).
Decaestecker, K. et al. Surveillance or metastasis-directed Therapy for OligoMetastatic Prostate cancer recurrence (STOMP): study protocol for a randomized phase II trial. BMC Cancer http://dx.doi.org/10.1186/1471-2407-14–671.
Crehange, G. et al. Salvage reirradiation for locoregional failure after radiation therapy for prostate cancer: Who, when, where and how? Cancer Radiother. 18, 524–534 (2014).
Valicenti, R. K. et al. Does hormone therapy reduce disease recurrence in prostate cancer patients receiving dose-escalated radiation therapy? An analysis of Radiation Therapy Oncology Group 94–06 Int. J. Radiat. Oncol. Biol. Phys. 79, 1323–1329 (2011).
McGuire, S. E. et al. PSA response to neoadjuvant androgen deprivation therapy is a strong independent predictor of survival in high-risk prostate cancer in the dose-escalated radiation therapy era. Int. J. Radiat. Oncol. Biol. Phys. 85, e39–e46 (2013).
Vergis, R. et al. Expression of Bcl-2, p53, and MDM2 in localized prostate cancer with respect to the outcome of radical radiotherapy dose escalation. Int. J. Radiat. Oncol. Biol. Phys. 78, 35–41 (2010).
Lilleby, W., Stensvold, A., Mills, I. G. & Nesland, J. M. Disseminated tumor cells and their prognostic significance in nonmetastatic prostate cancer patients. Int. J. Cancer 133, 149–155 (2013).
Jung, Y. et al. Prevalence of prostate cancer metastases after intravenous inoculation provides clues into the molecular basis of dormancy in the bone marrow microenvironment. Neoplasia 14, 429–439 (2012).
Murray, N. P. et al. Redefining micrometastasis in prostate cancer—a comparison of circulating prostate cells, bone marrow disseminated tumor cells and micrometastasis: Implications in determining local or systemic treatment for biochemical failure after radical prostatectomy. Int. J. Mol. Med. 30, 896–904 (2012).
Thalgott, M. et al. Detection of circulating tumor cells in different stages of prostate cancer. J. Cancer Res. Clin. Oncol. 139, 755–763 (2013).
Goodman, O. B. Jr et al. Circulating tumor cells as a predictive biomarker in patients with hormone-sensitive prostate cancer. Clin. Genitourin. Cancer 9, 31–38 (2011).
Morris, M. J. et al. Monitoring the clinical outcomes in advanced prostate cancer: what imaging modalities and other markers are reliable? Semin. Oncol. 40, 375–392 (2013).
Loh, J. et al. Circulating tumor cell detection in high-risk non-metastatic prostate cancer. J. Cancer Res. Clin. Oncol. 140, 2157–2162 (2014).
Newman, A. M. et al. An ultrasensitive method for quantitating circulating tumor DNA with broad patient coverage. Nat. Med. 20, 548–554 (2014).
Birkhauser, F. D. et al. Combined ultrasmall superparamagnetic particles of iron oxide-enhanced and diffusion-weighted magnetic resonance imaging facilitates detection of metastases in normal-sized pelvic lymph nodes of patients with bladder and prostate cancer. Eur. Urol. 64, 953–960 (2013).
Eiber, M. et al. Whole-body MRI including diffusion-weighted imaging (DWI) for patients with recurring prostate cancer: technical feasibility and assessment of lesion conspicuity in DWI. J. Magn. Reson. Imaging 33, 1160–1170 (2011).
Fox, J. J., Schoder, H. & Larson, S. M. Molecular imaging of prostate cancer. Curr. Opin. Urol. 22, 320–327 (2012).
Beauregard, J. M. & Pouliot, F. New developments in the imaging of metastatic prostate cancer. Curr. Opin. Support. Palliat. Care 8, 265–270 (2014).
Evangelista, L., Guttilla, A., Zattoni, F., Muzzio, P. C. & Zattoni, F. Utility of choline positron emission tomography/computed tomography for lymph node involvement identification in intermediate- to high-risk prostate cancer: a systematic literature review and meta-analysis. Eur. Urol. 63, 1040–1048 (2013).
Giovacchini, G. et al. 11C-choline PET/CT predicts prostate cancer-specific survival in patients with biochemical failure during androgen-deprivation therapy. J. Nucl. Med. 55, 233–241 (2014).
Sorensen, J., Owenius, R., Lax, M. & Johansson, S. Regional distribution and kinetics of [18F]fluciclovine (anti-[18F]FACBC), a tracer of amino acid transport, in subjects with primary prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 40, 394–402 (2013).
Afshar-Oromieh, A. et al. PET imaging with a [68Ga]gallium-labelled PSMA ligand for the diagnosis of prostate cancer: biodistribution in humans and first evaluation of tumour lesions. Eur. J. Nucl. Med. Mol. Imaging 40, 486–495 (2013).
Mansi, R., Fleischmann, A., Macke, H. R. & Reubi, J. C. Targeting GRPR in urological cancers—from basic research to clinical application. Nat. Rev. Urol. 10, 235–244 (2013).
Beattie, B. J. et al. Pharmacokinetic assessment of the uptake of 16beta-18F-fluoro-5alpha-dihydrotestosterone (FDHT) in prostate tumors as measured by PET. J. Nucl. Med. 51, 183–192 (2010).
Cuzick, J. et al. Prognostic value of a cell cycle progression signature for prostate cancer death in a conservatively managed needle biopsy cohort. Br. J. Cancer 106, 1095–1099 (2012).
Knezevic, D. et al. Analytical validation of the Oncotype DX prostate cancer assay - a clinical RT-PCR assay optimized for prostate needle biopsies. BMC Genomics http://dx.doi.org/10.1186/1471-2164-14–690.
Erho, N. et al. Discovery and validation of a prostate cancer genomic classifier that predicts early metastasis following radical prostatectomy. PLoS ONE http://dx.doi.org/10.1371/journal.pone.0066855.
McDunn, J. E. et al. Metabolomic signatures of aggressive prostate cancer. Prostate 73, 1547–1560 (2013).
Saylor, P. J., Karoly, E. D. & Smith, M. R. Prospective study of changes in the metabolomic profiles of men during their first three months of androgen deprivation therapy for prostate cancer. Clin. Cancer Res. 18, 3677–3685 (2012).
Jung, K. et al. Tissue metabolite profiling identifies differentiating and prognostic biomarkers for prostate carcinoma. Int. J. Cancer 133, 2914–2924 (2013).
Jin, R. et al. NF-kappaB gene signature predicts prostate cancer progression. Cancer Res. 74, 2763–2772 (2014).
Freedland, S. J. et al. Prognostic utility of cell cycle progression score in men with prostate cancer after primary external beam radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 86, 848–853 (2013).
Fraser, M., Berlin, A., Bristow, R. G. & van der Kwast, T. Genomic, pathological, and clinical heterogeneity as drivers of personalized medicine in prostate cancer. Urol. Oncol. http://dx.doi.org/10.1016/j.urolonc.2013.10.020.
Ranasinghe, W. K. et al. The role of hypoxia-inducible factor 1alpha in determining the properties of castrate-resistant prostate cancers. PLoS ONE http://dx.doi.org/10.1371/journal.pone.0054251.
Ranasinghe, W. K. et al. The effects of nonspecific HIF1alpha inhibitors on development of castrate resistance and metastases in prostate cancer. Cancer Med. 3, 245–251 (2014).
Al-Ubaidi, F. L. et al. Castration therapy results in decreased Ku70 levels in prostate cancer. Clin. Cancer Res. 19, 1547–1556 (2013).
Yapp, D. T. et al. Non-invasive evaluation of tumour hypoxia in the Shionogi tumour model for prostate cancer with 18F-EF5 and positron emission tomography. BJU Int. 99, 1154–1160 (2007).
Milosevic, M. et al. Tumor hypoxia predicts biochemical failure following radiotherapy for clinically localized prostate cancer. Clin. Cancer Res. 18, 2108–2114 (2012).
Bristow, R. G. & Hill, R. P. Hypoxia and metabolism. Hypoxia, DNA repair and genetic instability. Nat. Rev. Cancer 8, 180–192 (2008).
Luoto, K. R., Kumareswaran, R. & Bristow, R. G. Tumor hypoxia as a driving force in genetic instability. Genome Integr. 4, http://dx.doi.org/10.1186/2041-9414-4-5 (2013).
Chan, N., Milosevic, M. & Bristow, R. G. Tumor hypoxia, DNA repair and prostate cancer progression: new targets and new therapies. Future Oncol. 3, 329–341 (2007).
Milosevic, M. et al. Androgen withdrawal in patients reduces prostate cancer hypoxia: implications for disease progression and radiation response. Cancer Res. 67, 6022–6025 (2007).
Polkinghorn, W. R. et al. Androgen receptor signaling regulates DNA repair in prostate cancers. Cancer Discov. 3, 1245–1253 (2013).
Goodwin, J. F. et al. A hormone-DNA repair circuit governs the response to genotoxic insult. Cancer Discov. 3, 1254–1271 (2013).
Isaacs, J. T. et al. Androgen regulation of programmed death of normal and malignant prostatic cells. J. Androl. 13, 457–464 (1992).
Drake, C. G. Prostate cancer as a model for tumour immunotherapy. Nat. Rev. Immunol. 10, 580–593 (2010).
Roden, A. C. et al. Augmentation of T cell levels and responses induced by androgen deprivation. J. Immunol. 173, 6098–6108 (2004).
Kissick, H. T. et al. Androgens alter T-cell immunity by inhibiting T-helper 1 differentiation. Proc. Natl Acad. Sci. USA 111, 9887–9892 (2014).
Xie, B. X. et al. The radiation response of androgen-refractory prostate cancer cell line C4–2 derived from androgen-sensitive cell line LNCaP. Asian J. Androl. 12, 405–414 (2010).
Locke, J. A. et al. NKX3.1 haploinsufficiency is prognostic for prostate cancer relapse following surgery or image-guided radiotherapy. Clin. Cancer Res. 18, 308–316 (2012).
Won, A. C., Gurney, H., Marx, G., De Souza, P. & Patel, M. I. Primary treatment of the prostate improves local palliation in men who ultimately develop castrate-resistant prostate cancer. BJU Int. 112, E250–E255 (2013).
Pinkawa, M. et al. Local prostate cancer radiotherapy after prostate-specific antigen progression during primary hormonal therapy. Radiat. Oncol. http://dx.doi.org/10.1186/1748-717X-7–209.
Tabata, K. et al. Radiotherapy for oligometastases and oligo-recurrence of bone in prostate cancer. Pulm. Med. http://dx.doi.org/10.1155/2012/541656.
Bristow, R. G., Berlin, A. & Dal Pra, A. An arranged marriage for precision medicine: hypoxia and genomic assays in localized prostate cancer radiotherapy. Br. J. Radiol. http://dx.doi.org/10.1259/bjr.20130753.
Berlin, A. et al. Prognostic utility of cell cycle progession score in men with prostate cancer after primary external beam radiation therapy. In regard to Freedland et al. Int. J. Radiat. Oncol. Biol. Phys. 88, 237–240 (2014).
D'Amico, A. V. et al. Duration of short-course androgen suppression therapy and the risk of death as a result of prostate cancer. J. Clin. Oncol. 29, 4682–4687 (2011).
Khor, L. Y. et al. MDM2 and Ki-67 predict for distant metastasis and mortality in men treated with radiotherapy and androgen deprivation for prostate cancer: RTOG 92–02. J. Clin. Oncol. 27, 3177–3184 (2009).
Abdel-Wahab, M. et al. Influence of number of CAG repeats on local control in the RTOG 86–10 protocol. Am. J. Clin. Oncol. 29, 14–20 (2006).
Prensner, J. R. et al. The long noncoding RNA SChLAP1 promotes aggressive prostate cancer and antagonizes the SWI/SNF complex. Nat. Genet. 45, 1392–1398 (2013).
Lalonde, E. et al. Tumour genomic and microenvironmental heterogeneity for integrated prediction of 5-year biochemical recurrence of prostate cancer: a retrospective cohort study. Lancet Oncol. 15, 1521–1532 (2014).
Beltran, H. et al. Targeted next-generation sequencing of advanced prostate cancer identifies potential therapeutic targets and disease heterogeneity. Eur. Urol. 63, 920–926 (2013).
Marcelli, M. et al. Androgen receptor mutations in prostate cancer. Cancer Res. 60, 944–949 (2000).
Miyamoto, D. T., Sequist, L. V. & Lee, R. J. Circulating tumour cells-monitoring treatment response in prostate cancer. Nat. Rev. Clin. Oncol. 11, 401–412 (2014).
Miyamoto, D. T. et al. Androgen receptor signaling in circulating tumor cells as a marker of hormonally responsive prostate cancer. Cancer Discov. 2, 995–1003 (2012).
Mitsiades, N. et al. Distinct patterns of dysregulated expression of enzymes involved in androgen synthesis and metabolism in metastatic prostate cancer tumors. Cancer Res. 72, 6142–6152 (2012).
Lawton, C. A. et al. Updated results of the phase III Radiation Therapy Oncology Group (RTOG) trial 85–31 evaluating the potential benefit of androgen suppression following standard radiation therapy for unfavorable prognosis carcinoma of the prostate. Int. J. Radiat. Oncol. Biol. Phys. 49, 937–946 (2001).
Denham, J. W. et al. Short-term neoadjuvant androgen deprivation and radiotherapy for locally advanced prostate cancer: 10-year data from the TROG 96.01 randomised trial. Lancet Oncol. 12, 451–459 (2011).
Jones, C. U. et al. Radiotherapy and short-term androgen deprivation for localized prostate cancer. N. Engl. J. Med. 365, 107–108 (2011).
Bolla, M. Six months hormonal treatment in addition to radiotherapy improves survival for men with localised prostate cancer. The ASCO Post [online], (2014).
Hanks, G. E. et al. Phase III trial of long-term adjuvant androgen deprivation after neoadjuvant hormonal cytoreduction and radiotherapy in locally advanced carcinoma of the prostate: the Radiation Therapy Oncology Group Protocol 92–02. J. Clin. Oncol. 21, 3972–3978 (2003).
Widmark, A. et al. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. Lancet 373, 301–308 (2009).
Mottet, N., Peneau, M., Mazeron, J. J., Molinie, V. & Richaud, P. Addition of radiotherapy to long-term androgen deprivation in locally advanced prostate cancer: an open randomised phase 3 trial. Eur. Urol. 62, 213–219 (2012).
US National Library of Medicine. Clinicaltrials.gov [online], (2013).
US National Library of Medicine Clinicaltrials.gov [online], (2013).
US National Library of Medicine Clinicaltrials.gov [online], (2013).
US National Library of Medicine. Clinicaltrials.gov [online], (2013).
US National Library of Medicine Clinicaltrials.gov [online], (2013).
US National Library of Medicine Clinicaltrials.gov [online], (2013).
US National Library of Medicine Clinicaltrials.gov [online], (2014).
Davis, I. & Williams, S. Enzalutamide in androgen deprivation therapy with radiation therapy for high risk, clinically localised, prostate cancer: a randomised phase III trial. ANZUP [online], (2014).
US National Library of Medicine Clinicaltrials.gov [online], (2014).
Attard, G. et al. Combining Enzalutamide with Abiraterone, Prednisone, and Androgen Deprivation Therapy in the STAMPEDE Trial. Eur. Urol. [online], (2014).
Michaelson, D. Phase III Trial of Dose Escalated Radiation Therapy and Standard Androgen Deprivation Therapy (ADT) with a GnRH Agonist vs. Dose Escalated Radiation Therapy and Enhanced ADT with a GnRH Agonist and TAK-700 for Men with High Risk Prostate Cancer. Clinicaltrials.gov [online], (2014).
Claessens, F. et al. Emerging mechanisms of enzalutamide resistance in prostate cancer. Nat. Rev. Urol. 11, 712–716 (2014).
Rathkopf, D. E. et al. Phase I study of ARN-509, a novel antiandrogen, in the treatment of castration-resistant prostate cancer. J. Clin. Oncol. 31, 3525–3530 (2013).
Fizazi, K. et al. Activity and safety of ODM-201 in patients with progressive metastatic castration-resistant prostate cancer (ARADES): an open-label phase 1 dose-escalation and randomised phase 2 dose expansion trial. Lancet Oncol. 15, 975–985 (2014).
Giacinti, S. et al. Resistance to abiraterone in castration-resistant prostate cancer: a review of the literature. Anticancer Res. 34, 6265–6269 (2014).
D'Amico, A. V. et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 280, 969–974 (1998).
Author information
Authors and Affiliations
Contributions
J.A.L., A.D.P. and R.G.B. researched the data for the article. J.A.L. and R.G.B. contributed to discussions of the content of the article and contributed equally to writing the article. R.G.B. assisted with writing the article, and all authors contributed to reviewing and editing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Locke, J., Pra, A., Supiot, S. et al. Synergistic action of image-guided radiotherapy and androgen deprivation therapy. Nat Rev Urol 12, 193–204 (2015). https://doi.org/10.1038/nrurol.2015.50
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrurol.2015.50
This article is cited by
-
SPARKLE: a new spark in treating oligorecurrent prostate cancer: adding systemic treatment to stereotactic body radiotherapy or metastasectomy: key to long-lasting event-free survival?
BMC Cancer (2022)
-
microRNAs identified in prostate cancer: Correlative studies on response to ionizing radiation
Molecular Cancer (2020)
-
Using hormone therapy with salvage radiotherapy according to presalvage PSA levels
Nature Reviews Urology (2020)
-
The use of Hormonal Therapy to Augment Radiation Therapy in Prostate Cancer: An Update
Current Urology Reports (2017)