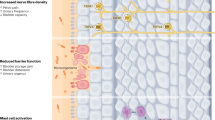
Key Points
-
Chronic pelvic pain syndrome (CPPS) is the most common classification of prostatitis and affects up to 16% of men at some point in their lives
-
CPPS is classified as an abacterial disease at time of diagnoses but acute bacterial infection might initiate inflammation and development of pain
-
Loss of regulation and suppression of CD4+ T-cell activity might result in constitutively active mast cells and loss of tolerance in CPPS
-
Mast-cell-released factors, such as tryptase and nerve growth factor, might mediate both inflammation and neural sensitization
-
CPPS has a complex aetiology, with evidence suggesting interplay between T cells, mast cells and neurons in the development of autoimmunity
Abstract
The cause of chronic pelvic pain syndrome (CPPS) has yet to be established. Since the late 1980s, cytokine, chemokine, and immunological classification studies using human samples have focused on identifying biomarkers for CPPS, but no diagnostically beneficial biomarkers have been identified, and these studies have done little to deepen our understanding of the mechanisms underlying chronic prostatic pain. Given the large number of men thought to be affected by this condition and the ineffective nature of current treatments, there is a pressing need to elucidate these mechanisms. Prostatitis types IIIa and IIIb are classified according to the presence of pain without concurrent presence of bacteria; however, it is becoming more evident that, although levels of bacteria are not directly associated with levels of pain, the presence of bacteria might act as the initiating factor that drives primary activation of mast-cell-mediated inflammation in the prostate. Mast cell activation is also known to suppress regulatory T cell (Treg) control of self-tolerance and also activate neural sensitization. This combination of established autoimmunity coupled with peripheral and central neural sensitization can result in the development of multiple symptoms, including pelvic pain and bladder irritation. Identifying these mechanisms as central mediators in CPPS offers new insight into the prospective treatment of the disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Krieger, J. N., Nyberg, L. Jr & Nickel, J. C. NIH consensus definition and classification of prostatitis. JAMA 282, 236–237 (1999).
Mahal, B. A. et al. The role of phenotyping in chronic prostatitis/chronic pelvic pain syndrome. Curr. Urol. Rep. 12, 297–303 (2011).
True, L. D., Berger, R. E., Rothman, I., Ross, S. O. & Krieger, J. N. Prostate histopathology and the chronic prostatitis/chronic pelvic pain syndrome: a prospective biopsy study. J. Urol. 162, 2014–2018 (1999).
Collins, M. M. et al. Prevalence and correlates of prostatitis in the health professionals follow-up study cohort. J. Urol. 167, 1363–1366 (2002).
Nickel, J. C., Downey, J., Hunter, D. & Clark, J. Prevalence of prostatitis-like symptoms in a population based study using the National Institutes of Health chronic prostatitis symptom index. J. Urol. 165, 842–845 (2001).
Tripp, D. A., Nickel, J. C., Ross, S., Mullins, C. & Stechyson, N. Prevalence, symptom impact and predictors of chronic prostatitis-like symptoms in Canadian males aged 16–19 years. BJU Int. 103, 1080–1084 (2009).
Ferris, J. A. et al. National prevalence of urogenital pain and prostatitis-like symptoms in Australian men using the National Institutes of Health Chronic Prostatitis Symptoms Index. BJU Int. 105, 373–379 (2010).
Liang, C. Z. et al. The prevalence of prostatitis-like symptoms in China. J. Urol. 182, 558–563 (2009).
Mehik, A., Hellstrom, P., Lukkarinen, O., Sarpola, A. & Jarvelin, M. Epidemiology of prostatitis in Finnish men: a population-based cross-sectional study. BJU Int. 86, 443–448 (2000).
Schaeffer, A. J. Epidemiology and evaluation of chronic pelvic pain syndrome in men. Int. J. Antimicrob. Agents 31 (Suppl. 1), S108–S111 (2008).
Ku, J. H., Kim, S. W. & Paick, J. S. Quality of life and psychological factors in chronic prostatitis/chronic pelvic pain syndrome. Urology 66, 693–701 (2005).
Konkle, K. S. & Clemens, J. Q. New paradigms in understanding chronic pelvic pain syndrome. Curr. Urol. Rep. 12, 278–283 (2011).
Pontari, M. & Giusto, L. New developments in the diagnosis and treatment of chronic prostatitis/chronic pelvic pain syndrome. Curr. Opin. Urol. 23, 565–569 (2013).
Schaeffer, A. J. Etiology and management of chronic pelvic pain syndrome in men. Urology 63, 75–84 (2004).
Motrich, R. D. et al. Reduced semen quality in chronic prostatitis patients that have cellular autoimmune response to prostate antigens. Hum. Reprod. 20, 2567–2572 (2005).
Wagenlehner, F. M. et al. National Institutes of Health Chronic Prostatitis Symptom Index (NIH-CPSI) symptom evaluation in multinational cohorts of patients with chronic prostatitis/chronic pelvic pain syndrome. Eur. Urol. 63, 953–959 (2013).
Walz, J. et al. Impact of chronic prostatitis-like symptoms on the quality of life in a large group of men. BJU Int. 100, 1307–1311 (2007).
Davis, S. N., Binik, Y. M., Amsel, R. & Carrier, S. A subtype-based analysis of urological chronic pelvic pain syndrome in men. J. Urol. 190, 118–123 (2013).
Le, B. V. & Schaeffer, A. J. Genitourinary pain syndromes, prostatitis, and lower urinary tract symptoms. Urol. Clin. North Am. 36, 527–536 (2009).
Anderson, R. U., Wise, D., Sawyer, T. & Chan, C. A. Sexual dysfunction in men with chronic prostatitis/chronic pelvic pain syndrome: improvement after trigger point release and paradoxical relaxation training. J. Urol. 176, 1534–1539 (2006).
Magri, V. et al. Use of the UPOINT chronic prostatitis/chronic pelvic pain syndrome classification in European patient cohorts: sexual function domain improves correlations. J. Urol. 184, 2339–2345 (2010).
Kaplan, S. A. et al. Etiology of voiding dysfunction in men less than 50 years of age. Urology 47, 836–839 (1996).
Kwon, J. K. & Chang, I. H. Pain, catastrophizing, and depression in chronic prostatitis/chronic pelvic pain syndrome. Int. Neurourol. J. 17, 48–58 (2013).
Anderson, R. U., Sawyer, T., Wise, D., Morey, A. & Nathanson, B. H. Painful myofascial trigger points and pain sites in men with chronic prostatitis/chronic pelvic pain syndrome. J. Urol. 182, 2753–2758 (2009).
Hedelin, H. H. Evaluation of a modification of the UPOINT clinical phenotype system for the chronic pelvic pain syndrome. Scand. J. Urol. Nephrol. 43, 373–376 (2009).
Shoskes, D. A., Nickel, J. C. & Kattan, M. W. Phenotypically directed multimodal therapy for chronic prostatitis/chronic pelvic pain syndrome: a prospective study using UPOINT. Urology 75, 1249–1253 (2010).
Nickel, J. C. & Shoskes, D. A. Phenotypic approach to the management of the chronic prostatitis/chronic pelvic pain syndrome. BJU Int. 106, 1252–1263 (2010).
Potts, J. M. & Payne, C. K. Urologic chronic pelvic pain. Pain 153, 755–758 (2012).
Tugcu, V. et al. A placebo-controlled comparison of the efficiency of triple- and monotherapy in category III B chronic pelvic pain syndrome (CPPS). Eur. Urol. 51, 1113–1118 (2007).
Baranowski, A. P. Urogenital/pelvic pain in men. Curr. Opin. Support. Palliat. Care 6, 213–219 (2012).
Rodriguez, M. A. et al. Evidence for overlap between urological and nonurological unexplained clinical conditions. J. Urol. 182, 2123–2131 (2009).
Samplaski, M. K., Li, J. & Shoskes, D. A. Clustering of UPOINT domains and subdomains in men with chronic prostatitis/chronic pelvic pain syndrome and contribution to symptom severity. J. Urol. 188, 1788–1793 (2012).
Shoskes, D. A., Prots, D., Karns, J., Horhn, J. & Shoskes, A. C. Greater endothelial dysfunction and arterial stiffness in men with chronic prostatitis/chronic pelvic pain syndrome—a possible link to cardiovascular disease. J. Urol. 186, 907–910 (2011).
Mendall, M. A. et al. Relation of Helicobacter pylori infection and coronary heart disease. Br. Heart J. 71, 437–439 (1994).
Khorasani, B., Arab, A. M., Sedighi Gilani, M. A., Samadi, V. & Assadi, H. Transabdominal ultrasound measurement of pelvic floor muscle mobility in men with and without chronic prostatitis/chronic pelvic pain syndrome. Urology 80, 673–677 (2012).
Rudick, C. N. et al. Host-pathogen interactions mediating pain of urinary tract infection. J. Infect. Dis. 201, 1240–1249 (2010).
Motrich, R. D., Cuffini, C., Oberti, J. P., Maccioni, M. & Rivero, V. E. Chlamydia trachomatis occurrence and its impact on sperm quality in chronic prostatitis patients. J. Infect. 53, 175–183 (2006).
Wagenlehner, F. M. et al. Bacterial prostatitis. World J. Urol. 31, 711–716 (2013).
Doble, A. et al. The role of Chlamydia trachomatis in chronic abacterial prostatitis: a study using ultrasound guided biopsy. J. Urol. 141, 332–333 (1989).
Doble, A. et al. A search for infectious agents in chronic abacterial prostatitis using ultrasound guided biopsy. Br. J. Urol. 64, 297–301 (1989).
Rudick, C. N. et al. Uropathogenic Escherichia coli induces chronic pelvic pain. Infect. Immun. 79, 628–635 (2011).
Sivick, K. E., Schaller, M. A., Smith, S. N. & Mobley, H. L. The innate immune response to uropathogenic Escherichia coli involves IL-17A in a murine model of urinary tract infection. J. Immunol. 184, 2065–2075 (2010).
Huang, B. R. et al. Interaction of inflammatory and anti-inflammatory responses in microglia by Staphylococcus aureus-derived lipoteichoic acid. Toxicol. Appl. Pharmacol. 269, 43–50 (2013).
Myles, I. A. et al. Signalling via the IL-20 receptor inhibits cutaneous production of IL-1beta and IL-17A to promote infection with methicillin-resistant Staphylococcus aureus. Nat. Immunol. 14, 804–811 (2013).
Karatas, O. F., Bayrak, O., Cimentepe, E. & Unal, D. An occult risk factor for chronic prostatitis: Helicobacter pylori. Med. Hypotheses 69, 963–964 (2007).
Karatas, O. F., Turkay, C., Bayrak, O., Cimentepe, E. & Unal, D. Helicobacter pylori seroprevalence in patients with chronic prostatitis: a pilot study. Scand. J. Urol. Nephrol. 44, 91–94 (2010).
Tomaskovic, I., Ruzic, B., Trnski, D. & Kraus, O. Chronic prostatitis/chronic pelvic pain syndrome in males may be an autoimmune disease, potentially responsive to corticosteroid therapy. Med. Hypotheses 72, 261–262 (2009).
Theyer, G. et al. Phenotypic characterization of infiltrating leucocytes in benign prostatic hyperplasia. Lab. Invest. 66, 96–107 (1992).
Steiner, G. et al. Phenotype and function of peripheral and prostatic lymphocytes in patients with benign prostatic hyperplasia. J. Urol. 151, 480–484 (1994).
Bierhoff, E. et al. Morphological analogies of fetal prostate stroma and stromal nodules in BPH. Prostate 31, 234–240 (1997).
Kramer, G. et al. Increased expression of lymphocyte-derived cytokines in benign hyperplastic prostate tissue, identification of the producing cell types, and effect of differentially expressed cytokines on stromal cell proliferation. Prostate 52, 43–58 (2002).
Quick, M. L. et al. Th1-Th17 cells contribute to the development of uropathogenic Escherichia coli-induced chronic pelvic pain. PLoS ONE 8, e60987 (2013).
Carlson, T., Kroenke, M., Rao, P., Lane, T. E. & Segal, B. The Th17-ELR+ CXC chemokine pathway is essential for the development of central nervous system autoimmune disease. J. Exp. Med. 205, 811–823 (2008).
Zhang, H. L., Zheng, X. Y. & Zhu, J. Th1/Th2/Th17/Treg cytokines in Guillain-Barre syndrome and experimental autoimmune neuritis. Cytokine Growth Factor Rev. 24, 443–453 (2013).
Zielinski, C. E. et al. Pathogen-induced human TH17 cells produce IFN-gamma or IL-10 and are regulated by IL-1beta. Nature 484, 514–518 (2012).
Motrich, R. D., Maccioni, M., Riera, C. M. & Rivero, V. E. Autoimmune prostatitis: state of the art. Scand. J. Immunol. 66, 217–227 (2007).
Rudick, C. N., Schaeffer, A. J. & Thumbikat, P. Experimental autoimmune prostatitis induces chronic pelvic pain. Am. J. Physiol. Regul. Integr. Comp. Physiol. 294, R1268–R1275 (2008).
Maccioni, M., Rivero, V. E. & Riera, C. M. Prostatein (or rat prostatic steroid binding protein) is a major autoantigen in experimental autoimmune prostatitis. Clin. Exp. Immunol. 112, 159–165 (1998).
Rivero, V., Carnaud, C. & Riera, C. M. Prostatein or steroid binding protein (PSBP) induces experimental autoimmune prostatitis (EAP) in NOD mice. Clin. Immunol. 105, 176–184 (2002).
Penna, G. et al. Spontaneous and prostatic steroid binding protein peptide-induced autoimmune prostatitis in the nonobese diabetic mouse. J. Immunol. 179, 1559–1567 (2007).
Rivero, V. E., Cailleau, C., Depiante-Depaoli, M., Riera, C. M. & Carnaud, C. Non-obese diabetic (NOD) mice are genetically susceptible to experimental autoimmune prostatitis (EAP). J. Autoimmun. 11, 603–610 (1998).
Louvet, C. et al. Tyrosine kinase inhibitors reverse type 1 diabetes in nonobese diabetic mice. Proc. Natl Acad. Sci. USA 105, 18895–18900 (2008).
Gallegos, A. M. & Bevan, M. J. Driven to autoimmunity: the nod mouse. Cell 117, 149–151 (2004).
Kim, J. M. & Rudensky, A. The role of the transcription factor Foxp3 in the development of regulatory T cells. Immunol. Rev. 212, 86–98 (2006).
Bonomo, A., Kehn, P. J. & Shevach, E. M. Post-thymectomy autoimmunity: abnormal T-cell homeostasis. Immunol. Today 16, 61–67 (1995).
Taguchi, O. & Nishizuka, Y. Self tolerance and localized autoimmunity. Mouse models of autoimmune disease that suggest tissue-specific suppressor T cells are involved in self tolerance. J. Exp. Med. 165, 146–156 (1987).
Gallegos, A. M. & Bevan, M. J. Central tolerance: good but imperfect. Immunol. Rev. 209, 290–296 (2006).
Gallegos, A. M. & Bevan, M. J. Central tolerance to tissue-specific antigens mediated by direct and indirect antigen presentation. J. Exp. Med. 200, 1039–1049 (2004).
Anderson, M. S. et al. Projection of an immunological self shadow within the thymus by the Aire protein. Science 298, 1395–1401 (2002).
Rizzi, M., Ferrera, F., Filaci, G. & Indiveri, F. Disruption of immunological tolerance: role of AIRE gene in autoimmunity. Autoimmun. Rev. 5, 145–147 (2006).
Ferrera, F. et al. AIRE gene polymorphisms in systemic sclerosis associated with autoimmune thyroiditis. Clin. Immunol. 122, 13–17 (2007).
Anderson, M. S. et al. The cellular mechanism of Aire control of T cell tolerance. Immunity 23, 227–239 (2005).
Villasenor, J., Benoist, C. & Mathis, D. AIRE and APECED: molecular insights into an autoimmune disease. Immunol. Rev. 204, 156–164 (2005).
Kim, J. M., Rasmussen, J. P. & Rudensky, A. Y. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat. Immunol. 8, 191–197 (2007).
Taguchi, O., Kojima, A. & Nishizuka, Y. Experimental autoimmune prostatitis after neonatal thymectomy in the mouse. Clin. Exp. Immunol. 60, 123–129 (1985).
Bonomo, A. et al. Pathogenesis of post-thymectomy autoimmunity. Role of syngeneic MLR-reactive T cells. J. Immunol. 154, 6602–6611 (1995).
Setiady, Y. Y. et al. Physiologic self antigens rapidly capacitate autoimmune disease-specific polyclonal CD4+ CD25+ regulatory T cells. Blood 107, 1056–1062 (2006).
de Vries, V. C. et al. Mast cell degranulation breaks peripheral tolerance. Am. J. Transplant. 9, 2270–2280 (2009).
Shoskes, D. A., Albakri, Q., Thomas, K. & Cook, D. Cytokine polymorphisms in men with chronic prostatitis/chronic pelvic pain syndrome: association with diagnosis and treatment response. J. Urol. 168, 331–335 (2002).
Shoskes, D. A. & Nickel, J. C. Quercetin for chronic prostatitis/chronic pelvic pain syndrome. Urol. Clin. North Am. 38, 279–284 (2011).
John, H. et al. Noninflammatory chronic pelvic pain syndrome: immunological study in blood, ejaculate and prostate tissue. Eur. Urol. 39, 72–78 (2001).
Penna, G. et al. Seminal plasma cytokines and chemokines in prostate inflammation: interleukin 8 as a predictive biomarker in chronic prostatitis/chronic pelvic pain syndrome and benign prostatic hyperplasia. Eur. Urol. 51, 524–533 (2007).
Orhan, I., Onur, R., Ilhan, N. & Ardicoglu, A. Seminal plasma cytokine levels in the diagnosis of chronic pelvic pain syndrome. Int. J. Urol. 8, 495–499 (2001).
Motrich, R. D. et al. Pathogenic consequences in semen quality of an autoimmune response against the prostate gland: from animal models to human disease. J. Immunol. 177, 957–967 (2006).
Khadra, A., Fletcher, P., Luzzi, G., Shattock, R. & Hay, P. Interleukin-8 levels in seminal plasma in chronic prostatitis/chronic pelvic pain syndrome and nonspecific urethritis. BJU Int. 97, 1043–1046 (2006).
John, H. et al. Immunological alterations in the ejaculate of chronic prostatitis patients: clues for autoimmunity. Andrologia 35, 294–299 (2003).
Ludwig, M. et al. Immunocytological analysis of leucocyte subpopulations in urine specimens before and after prostatic massage. Eur. Urol. 39, 277–282 (2001).
Nickel, J. C. et al. Leucocytes and bacteria in men with chronic prostatitis/chronic pelvic pain syndrome compared to asymptomatic controls. J. Urol. 170, 818–822 (2003).
Breser, M. L., Motrich, R. D., Sanchez, L. R., Mackern-Oberti, J. P. & Rivero, V. E. Expression of CXCR3 on specific T cells is essential for homing to the prostate gland in an experimental model of chronic prostatitis/chronic pelvic pain syndrome. J. Immunol. 190, 3121–3133 (2013).
Batstone, G. R., Doble, A. & Gaston, J. S. Autoimmune T cell responses to seminal plasma in chronic pelvic pain syndrome (CPPS). Clin. Exp. Immunol. 128, 302–307 (2002).
Kouiavskaia, D. V., Southwood, S., Berard, C. A., Klyushnenkova, E. N. & Alexander, R. B. T-cell recognition of prostatic peptides in men with chronic prostatitis/chronic pelvic pain syndrome. J. Urol. 182, 2483–2489 (2009).
Motrich, R. D. et al. Presence of INFγ-secreting lymphocytes specific to prostate antigens in a group of chronic prostatitis patients. Clin. Immunol. 116, 149–157 (2005).
Ponniah, S., Arah, I. & Alexander, R. B. PSA is a candidate self-antigen in autoimmune chronic prostatitis/chronic pelvic pain syndrome. Prostate 44, 49–54 (2000).
Alexander, R. B., Brady, F., Leffell, M. S., Tsai, V. & Celis, E. Specific T cell recognition of peptides derived from prostate-specific antigen in patients with prostate cancer. Urology 51, 150–157 (1998).
Doble, A., Walker, M. M., Harris, J. R., Taylor-Robinson, D. & Witherow, R. O. Intraprostatic antibody deposition in chronic abacterial prostatitis. Br. J. Urol. 65, 598–605 (1990).
Thumbikat, P. et al. Prostate secretions from men with chronic pelvic pain syndrome inhibit proinflammatory mediators. J. Urol. 184, 1536–1542 (2010).
Jung, H., Toth, P. T., White, F. A. & Miller, R. J. Monocyte chemoattractant protein-1 functions as a neuromodulator in dorsal root ganglia neurons. J. Neurochem. 104, 254–263 (2008).
Van Steenwinckel, J. et al. CCL2 released from neuronal synaptic vesicles in the spinal cord is a major mediator of local inflammation and pain after peripheral nerve injury. J. Neurosci. 31, 5865–5875 (2011).
Koch, A. E. et al. Macrophage inflammatory protein-1 alpha. A novel chemotactic cytokine for macrophages in rheumatoid arthritis. J. Clin. Invest. 93, 921–928 (1994).
Koch, A. E. et al. Enhanced production of the chemotactic cytokines interleukin-8 and monocyte chemoattractant protein-1 in human abdominal aortic aneurysms. Am. J. Pathol. 142, 1423–1431 (1993).
Ikeda, U., Matsui, K., Murakami, Y. & Shimada, K. Monocyte chemoattractant protein-1 and coronary artery disease. Clin. Cardiol. 25, 143–147 (2002).
Nishimura, T. et al. Study of macrophages in prostatic fluid from nonbacterial prostatitis patients: Relation between activation of macrophages and stage of prostatitis. Urol. Int. 46, 15–17 (1991).
Zhang, N., Rogers, T. J., Caterina, M. & Oppenheim, J. J. Proinflammatory chemokines, such as C-C chemokine ligand 3, desensitize mu-opioid receptors on dorsal root ganglia neurons. J. Immunol. 173, 594–599 (2004).
Zhang, N. et al. A proinflammatory chemokine, CCL3, sensitizes the heat- and capsaicin-gated ion channel TRPV1. Proc. Natl Acad. Sci. USA 102, 4536–4541 (2005).
Desireddi, N. V. et al. Monocyte chemoattractant protein-1 and macrophage inflammatory protein-1α as possible biomarkers for the chronic pelvic pain syndrome. J. Urol. 179, 1857–1862 (2008).
White, F. A., Jung, H. & Miller, R. J. Chemokines and the pathophysiology of neuropathic pain. Proc. Natl Acad. Sci. USA 104, 20151–20158 (2007).
Quick, M. L. et al. CCL2 and CCL3 are essential mediators of pelvic pain in experimental autoimmune prostatitis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 303, R580–R589 (2012).
Heninger, A. K. et al. IL-7 abrogates suppressive activity of human CD4+CD25+FOXP3+ regulatory T cells and allows expansion of alloreactive and autoreactive T cells. J. Immunol. 189, 5649–5658 (2012).
Sayed, B. A., Christy, A., Quirion, M. R. & Brown, M. A. The master switch: the role of mast cells in autoimmunity and tolerance. Ann. Rev. Immunol. 26, 705–739 (2008).
Walker, M. E., Hatfield, J. K. & Brown, M. A. New insights into the role of mast cells in autoimmunity: evidence for a common mechanism of action? Biochim. Biophys. Acta 1822, 57–65 (2012).
Kalesnikoff, J. & Galli, S. J. New developments in mast cell biology. Nat. Immunol. 9, 1215–1223 (2008).
Metz, M. & Maurer, M. Mast cells--key effector cells in immune responses. Trends Immunol. 28, 234–241 (2007).
Rao, K. N. & Brown, M. A. Mast cells: multifaceted immune cells with diverse roles in health and disease. Ann. NY Acad. Sci. 1143, 83–104 (2008).
Hallgren, J. & Pejler, G. Biology of mast cell tryptase. An inflammatory mediator. FEBS J. 273, 1871–1895 (2006).
Kim, D. Y., Jeoung, D. & Ro, J. Y. Signalling pathways in the activation of mast cells cocultured with astrocytes and co-localization of both cells in experimental allergic encephalomyelitis. J. Immunol. 185, 273–283 (2010).
Sayed, B. A., Christy, A. L., Walker, M. E. & Brown, M. A. Meningeal mast cells affect early T cell central nervous system infiltration and blood-brain barrier integrity through TNF: a role for neutrophil recruitment? J. Immunol. 184, 6891–6900 (2010).
Brenner, T., Soffer, D., Shalit, M. & Levi-Schaffer, F. Mast cells in experimental allergic encephalomyelitis: characterization, distribution in the CNS and in vitro activation by myelin basic protein and neuropeptides. J. Neurol. Sci. 122, 210–213 (1994).
Yeh, W. I., McWilliams, I. L. & Harrington, L. E. Autoreactive Tbet-positive CD4 T cells develop independent of classic Th1 cytokine signalling during experimental autoimmune encephalomyelitis. J. Immunol. 187, 4998–5006 (2011).
Fletcher, J. M., Lalor, S. J., Sweeney, C. M., Tubridy, N. & Mills, K. H. T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin. Exp. Immunol. 162, 1–11 (2010).
Murphy, A. C., Lalor, S. J., Lynch, M. A. & Mills, K. H. Infiltration of Th1 and Th17 cells and activation of microglia in the CNS during the course of experimental autoimmune encephalomyelitis. Brain Behav. Immun. 24, 641–651 (2010).
Eller, K. et al. IL-9 production by regulatory T cells recruits mast cells that are essential for regulatory T cell-induced immune suppression. J. Immunol. 186, 83–91 (2011).
Steinman, L. Multiple sclerosis: a coordinated immunological attack against myelin in the central nervous system. Cell 85, 299–302 (1996).
Tanzola, M. B., Robbie-Ryan, M., Gutekunst, C. A. & Brown, M. A. Mast cells exert effects outside the central nervous system to influence experimental allergic encephalomyelitis disease course. J. Immunol. 171, 4385–4391 (2003).
Thompson, A. J. et al. Patterns of disease activity in multiple sclerosis: clinical and magnetic resonance imaging study. BMJ 300, 631–634 (1990).
Johnson, D., Seeldrayers, P. A. & Weiner, H. L. The role of mast cells in demyelination. 1. Myelin proteins are degraded by mast cell proteases and myelin basic protein and P2 can stimulate mast cell degranulation. Brain Res. 444, 195–198 (1988).
Piconese, S. et al. Exacerbated experimental autoimmune encephalomyelitis in mast-cell-deficient Kit W-sh/W-sh mice. Lab. Invest. 91, 627–641 (2011).
Lee, D. M. et al. Mast cells: a cellular link between autoantibodies and inflammatory arthritis. Science 297, 1689–1692 (2002).
Shin, K. et al. Mast cells contribute to autoimmune inflammatory arthritis via their tryptase/heparin complexes. J. Immunol. 182, 647–656 (2009).
Sawamukai, N. et al. Mast cell-derived tryptase inhibits apoptosis of human rheumatoid synovial fibroblasts via rho-mediated signalling. Arthritis Rheum. 62, 952–959 (2010).
Hueber, A. J. et al. Mast cells express IL-17A in rheumatoid arthritis synovium. J. Immunol. 184, 3336–3340 (2010).
Sandler, C. et al. Selective activation of mast cells in rheumatoid synovial tissue results in production of TNF-α, IL-1β and IL-1Ra. Inflamm. Res. 56, 230–239 (2007).
Brown, M. A., Sayed, B. A. & Christy, A. Mast cells and the adaptive immune response. J. Clin. Immunol. 28, 671–676 (2008).
Hong, G. U., Kim, N. G., Jeoung, D. & Ro, J. Y. Anti-CD40 Ab- or 8-oxo-dG-enhanced Treg cells reduce development of experimental autoimmune encephalomyelitis via downregulating migration and activation of mast cells. J. Neuroimmunol. 260, 60–73 (2013).
Piconese, S. et al. Mast cells counteract regulatory T-cell suppression through interleukin-6 and OX40/OX40L axis toward Th17-cell differentiation. Blood 114, 2639–2648 (2009).
Forward, N. A., Furlong, S. J., Yang, Y., Lin, T. J. & Hoskin, D. W. Mast cells downregulate CD4+CD25+ T regulatory cell suppressor function via histamine H1 receptor interaction. J. Immunol. 183, 3014–3022 (2009).
Ganeshan, K. & Bryce, P. J. Regulatory T cells enhance mast cell production of IL-6 via surface-bound TGF-β. J. Immunol. 188, 594–603 (2012).
Rivero, V. E., Iribarren, P. & Riera, C. M. Mast cells in accessory glands of experimentally induced prostatitis in male Wistar rats. Clin. Immunol. Immunopathol. 74, 236–242 (1995).
Grimbaldeston, M. A. et al. Mast cell-deficient W-sash c-kit mutant Kit W-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am. J. Pathol. 167, 835–848 (2005).
Palmer, H. S. et al. Protease-activated receptor 2 mediates the proinflammatory effects of synovial mast cells. Arthritis Rheum. 56, 3532–3540 (2007).
Bali, K. K. et al. Transcriptional mechanisms underlying sensitization of peripheral sensory neurons by Granulocyte-/Granulocyte-macrophage colony stimulating factors. Mol. Pain 9, 48 (2013).
Okuse, K. Pain signalling pathways: from cytokines to ion channels. Int. J. Biochem. Cell Biol. 39, 490–496 (2007).
Thacker, M. A., Clark, A. K., Marchand, F. & McMahon, S. B. Pathophysiology of peripheral neuropathic pain: immune cells and molecules. Anesth. Analg. 105, 838–847 (2007).
Pezet, S. & McMahon, S. B. Neurotrophins: mediators and modulators of pain. Annu. Rev. Neurosci. 29, 507–538 (2006).
Watanabe, T. et al. Nerve growth factor level in the prostatic fluid of patients with chronic prostatitis/chronic pelvic pain syndrome is correlated with symptom severity and response to treatment. BJU Int. 108, 248–251 (2011).
Woolf, C. J. & Salter, M. W. Neuronal plasticity: increasing the gain in pain. Science 288, 1765–1769 (2000).
Author information
Authors and Affiliations
Contributions
S.F.M. and P.T. researched the literature, wrote, edited, and reviewed the article. A.J.S. made substantial contributions towards discussions of contents and reviewed the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
PowerPoint slides
Rights and permissions
About this article
Cite this article
Murphy, S., Schaeffer, A. & Thumbikat, P. Immune mediators of chronic pelvic pain syndrome. Nat Rev Urol 11, 259–269 (2014). https://doi.org/10.1038/nrurol.2014.63
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrurol.2014.63
This article is cited by
-
Comparing the effectiveness of extracorporeal shockwave therapy and myofascial release therapy in chronic pelvic pain syndrome: study protocol for a randomized controlled trial
Trials (2023)
-
The efficacy and safety of low-intensity extracorporeal shock wave treatment combined with or without medications in Chronic prostatitis/chronic pelvic pain syndrome: a systematic review and meta-analysis
Prostate Cancer and Prostatic Diseases (2023)
-
Radial Extracorporeal Shock Wave Therapy Combined with Resveratrol Derivative Alleviates Chronic Nonbacterial Prostatitis in Rats
Inflammation (2023)
-
RETRACTED ARTICLE: Morphological study of chronic prostatitis–chronic pelvic pain syndrome (CP/CPPS) normal modeling dose of T2 peptide in mice
International Urology and Nephrology (2022)
-
IL-1β-primed mesenchymal stromal cells exert enhanced therapeutic effects to alleviate Chronic Prostatitis/Chronic Pelvic Pain Syndrome through systemic immunity
Stem Cell Research & Therapy (2021)