Abstract
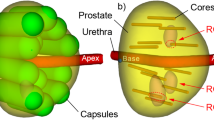
The current protocol for detecting and ruling out prostate cancer involves serum PSA testing followed by sampling of the prostate using a transrectal ultrasonography (TRUS)-guided biopsy. Many specialists have discussed how PSA screening has contributed to underdetection of clinically significant prostate cancer, overdiagnosis of clinically insignificant disease and poor risk stratification; however, little consideration has been given to the role of TRUS-guided biopsy in these errors. The performance of TRUS-guided biopsy is constrained by the biomechanical attributes of the sampling strategy, resulting in suboptimal detection efficiency of each core. By using a biomedical engineering approach, a uniform grid sampling strategy could be used to improve the detection efficiency of prostate biopsy. Moreover, the calibration of the sampling can be adjusted by altering the distance between needle deployments. Our model shows that for any given number of needle trajectories, a uniform grid approach will be superior to a divergent, nonuniform strategy for the detection of clinically important disease. This is an important message that should result in a move away from divergent sampling to a uniform grid approach for prostate biopsy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Strope, S. A. & Andriole, G. L. Prostate cancer screening: current status and future perspectives. Nat. Rev. Urol. 7, 487–493 (2010).
Esserman, L. & Thompson, I. Solving the overdiagnosis dilemma. J. Natl Cancer Inst. 102, 582–583 (2010).
Onik, G., Miessau, M. & Bostwick, D. G. Three-dimensional prostate mapping biopsy has a potentially significant impact on prostate cancer management. J. Clin. Oncol. 27, 4321–4326 (2009).
Taira, A. V. et al. Performance of transperineal template-guided mapping biopsy in detecting prostate cancer in the initial and repeat biopsy setting. Prostate Cancer Prostatic Dis. 13, 71–77 (2010).
Barqawi, A. B. et al. The role of 3-dimensional mapping biopsy in decision making for treatment of apparent early stage prostate cancer. J. Urol. 186, 80–85 (2011).
Schröder, F. H. et al. ERSPC Investigators. Screening and prostate-cancer mortality in a randomized European study. N. Engl. J. Med. 360, 1320–1328 (2009).
Hugosson, J. et al. Mortality results from the Göteborg randomised population-based prostate-cancer screening trial. Lancet Oncol. 11, 725–732 (2010).
Andriole, G. L. et al. REDUCE Study Group. Effect of dutasteride on the risk of prostate cancer. N. Engl. J. Med. 362, 1192–1202 (2010).
Heidenreich, A. et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur. Urol. 59, 61–71 (2011).
Brossner, C. et al. Distribution of prostate carcinoma foci within the peripheral zone: analysis of 8062 prostate biopsy cores. World J. Urol. 21, 163–166 (2003).
Djavan, B. & Margreiter, M. Biopsy standards for detection of prostate cancer. World J. Urol. 25, 11–17 (2007).
Kepner, G. & Kepner, J. Transperineal biopsy: analysis of a uniform core sampling pattern that yields data on tumor volume limits in negative biopsies. Theor. Biol. Med. Model. 7, 23 (2010).
Wise, A. M., Stamey, T. A., McNeal, J. E. & Clayton, J. L. Morphologic and clinical significance of multifocal prostate cancers in radical prostatectomy specimens. Urology 60, 264–269 (2002).
Ahmed, H. U. The index lesion and the origin of prostate cancer. N. Engl. J. Med. 361, 1704–1706 (2009).
Liu, W. et al. Copy number analysis indicates monoclonal origin of lethal metastatic prostate cancer. Nat. Med. 15, 559–565 (2009).
Netto, G. Tumor volume threshold of insignificant prostate cancer—was Dr. Stamey right all along? J. Urol. 185, 10–11 (2011).
Ploussard, G. et al. The contemporary concept of significant versus insignificant prostate cancer. Eur. Urol. 60, 291–303 (2011).
Stamey, T. et al. Localized prostate cancer. Relationship of tumor volume to clinical significance for treatment of prostate cancer. Cancer 71, 933–938 (1993).
Karavitakis, M., Ahmed, H. U., Abel, P. D., Hazell, S. & Winkler, M. H. Tumor focality in prostate cancer: implications for focal therapy. Nat. Rev. Clin. Oncol. 8, 48–55 (2011).
Ahmed, H. U. et al. Characterizing clinically significant prostate cancer using template prostate mapping biopsy. J. Urol. 186, 458–464 (2011).
Bouye, S. et al. Transition zone and anterior stromal prostate cancers: zone of origin and intraprostatic patterns of spread at histopathology. Prostate 69, 105–113 (2009).
Haffner, J. et al. Peripheral zone prostate cancers: location and intraprostatic patterns of spread at histopathology. Prostate 69, 276–282 (2009).
Delongchamps, N. & Hass, G. Saturation biopsies for prostate cancer: current uses and future prospects. Nat. Rev. Urol. 6, 645–652 (2009).
Patel, A. & Jones, S. Optimal biopsy strategies for the diagnosis and staging of prostate cancer. Curr. Opin. Urol. 19, 232–237 (2009).
Scattoni, V. et al. Biopsy schemes with the fewest cores for detecting 95% of the prostate cancers detected by a 24-core biopsy. Eur. Urol. 57, 1–8 (2010).
Singh, P., Ahmed, H. U. & Emberton, M. Active surveillance: Is there a need for better risk stratification at the outset? J. Clin. Oncol. 28, e513 (2010).
Wei, J. Limitations of a contemporary prostate biopsy: The blind march forward. Urol. Oncol. 28, 546–549 (2010).
Barzell, W. E. & Melamed, M. R. Appropriate patient selection in the focal treatment of prostate cancer: the role of transperineal 3-dimensional pathologic mapping of the prostate--a 4-year experience. Urology 70 (Suppl. 6), 27–35 (2007).
Megwalu, I. I. et al. Evaluation of a novel precision template-guided biopsy system for detecting prostate cancer. BJU Int. 102, 546–550 (2008).
Ukimura, O., Hung, A. & Gill, I. Innovations in prostate biopsy strategies for active surveillance and focal therapy. Curr. Opin. Urol. 21, 115–120 (2011).
Ahmed, H. U. et al. Is it time to consider a role for MRI before prostate biopsy? Nat. Rev. Clin. Oncol. 6, 197–206 (2009).
Mikolajczyk, S. D., Song, Y., Wong, J. R., Matson, R. S. & Rittenhouse, H. G. Are multiple markers the future of prostate cancer diagnostics? Clin. Biochem. 37, 519–528 (2004).
Acknowledgements
H. U. Ahmed and M. Emberton receive funding from the Medical Research Council, National Institute of Health Research-Health Technology Assessment programme, Pelican Cancer Foundation, Prostate Action, St Peter's Trust, Prostate Cancer Research Foundation, Prostate Cancer Charity and Prostate Cancer Research Centre. Mark Emberton receives funding in part from the UK National Institute of Health Research UCLH/UCL Comprehensive Biomedical Research Centre.
Author information
Authors and Affiliations
Contributions
H. U. Ahmed and G. Kepner researched data for the article. All authors contributed to discussion of content, writing the manuscript, and review and editing of the article before submission.
Corresponding author
Ethics declarations
Competing interests
H. U. Ahmed and M. Emberton receive funding from USHIFU, GSK and Advanced Medical Diagnostics for clinical trials, are paid consultants to Steba Biotech, and have received funding from USHIFU/Focused Surgery/Misonix Inc/UKHIFU (manufacturers and distributors of the Sonablate500 HIFU device) and Oncura for medical consultancy and travel to conferences. None of the funding sources had any role in the writing of this article. The other authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Ahmed, H., Emberton, M., Kepner, G. et al. A biomedical engineering approach to mitigate the errors of prostate biopsy. Nat Rev Urol 9, 227–231 (2012). https://doi.org/10.1038/nrurol.2012.3
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrurol.2012.3
This article is cited by
-
Likelihood of sampling prostate cancer at systematic biopsy as a function of gland volume and number of cores
Prostate Cancer and Prostatic Diseases (2024)
-
Multi-parametric MRI zone-specific diagnostic model performance compared with experienced radiologists for detection of prostate cancer
European Radiology (2019)
-
“Textural analysis of multiparametric MRI detects transition zone prostate cancer”
European Radiology (2017)
-
Targeted prostate biopsy and MR-guided therapy for prostate cancer
Abdominal Radiology (2016)
-
Targeted transperineal biopsy of the prostate has limited additional benefit over background cores for larger MRI-identified tumors
World Journal of Urology (2016)