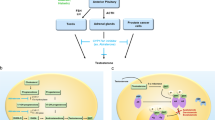
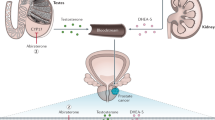
Abstract
The development of novel therapies such as abiraterone acetate and sipuleucel-T has improved the outlook for patients with advanced-stage and castration-resistant prostate cancer. However, the beneficial effects of these drugs are only measured in months. Moreover, the National Institute for Health and Clinical Excellence in the UK had ruled that the use of abiraterone acetate was not cost-effective before cost revision by the manufacturers. The FDA statement asserting that the use of 5α-reductase inhibitors for prostate cancer chemoprevention could increase the risk of developing high-grade prostate cancer also indirectly questions the value of direct androgen response manipulation for long-term benefit. These reports illustrate the need for a fresh and comprehensive analysis of advanced prostate cancer pathology to promote the next generation of effective adjuvant therapies. One such avenue is that of differentiation therapy, which seeks to promote the differentiation of cancer stem cells into a phenotype more sensitive to anticancer therapy than their parents. Using differentiation therapy with current antiandrogen therapies should augment our armoury of treatment for the management of advanced prostate cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Siegel, R., Naishadham, D. & Jemal, A. Cancer statistics, 2012. CA Cancer J. Clin. 62, 10–29 (2012).
Carducci, M. A. et al. A phase 3 randomized controlled trial of the efficacy and safety of atrasentan in men with metastatic hormone-refractory prostate cancer. Cancer 110, 1959–1966 (2007).
Sternberg, C. N. et al. Multinational, double-blind, phase III study of prednisone and either satraplatin or placebo in patients with castrate-refractory prostate cancer progressing after prior chemotherapy: the SPARC trial. J. Clin. Oncol. 27, 5431–5438 (2009).
Saad, F. et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J. Natl Cancer Inst. 94, 1458–1468 (2002).
Petrylak, D. P. et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N. Engl. J. Med. 351, 1513–1520 (2004).
Tannock, I. F. et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N. Engl. J. Med. 351, 1502–1512 (2004).
Kantoff, P. W. et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 363, 411–422 (2010).
Attard, G. et al. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J. Clin. Oncol. 27, 3742–3748 (2009).
Scher, H. I. et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1–2 study. Lancet 375, 1437–1446 (2010).
Ning, Y. M. et al. Phase II trial of bevacizumab, thalidomide, docetaxel, and prednisone in patients with metastatic castration-resistant prostate cancer. J. Clin. Oncol. 28, 2070–2076 (2010).
Nilsson, S. et al. Bone-targeted radium-223 in symptomatic, hormone-refractory prostate cancer: a randomised, multicentre, placebo-controlled phase II study. Lancet Oncol. 8, 587–594 (2007).
Yap, T. A., Zivi, A., Omlin, A. & de Bono, J. S. The changing therapeutic landscape of castration-resistant prostate cancer. Nat. Rev. Clin. Oncol. 8, 597–610 (2011).
de Bono, J. S. et al. Abiraterone and increased survival in metastatic prostate cancer. N. Engl. J. Med. 364, 1995–2005 (2011).
Beltran, H. et al. New therapies for castration-resistant prostate cancer: efficacy and safety. Eur. Urol. 60, 279–290 (2011).
National Institute for Health and Clinical Excellence. NICE consults on a new treatment for prostate cancer of article [online], (2012).
Thompson, I. M. et al. The influence of finasteride on the development of prostate cancer. N. Engl. J. Med. 349, 215–224 (2003).
Andriole, G. L. et al. Effect of dutasteride on the risk of prostate cancer. N. Engl. J. Med. 362, 1192–1202 (2010).
FDA. FDA drug safety communication: 5α reductase inhibitors (5-ARIs) may increase the risk of a more serious form of prostate cancer [online], (2011).
Horning, S. J. Natural history of and therapy for the indolent non-Hodgkin's lymphomas. Semin. Oncol. 20, 75–88 (1993).
Child, J. A. et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N. Engl. J. Med. 348, 1875–1883 (2003).
Rocha Lima, C. M. et al. Irinotecan plus gemcitabine results in no survival advantage compared with gemcitabine monotherapy in patients with locally advanced or metastatic pancreatic cancer despite increased tumor response rate. J. Clin. Oncol. 22, 3776–3783 (2004).
Ben-Porath, I. et al. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat. Genet. 40, 499–507 (2008).
Stevenson, M. et al. Characterizing the clinical relevance of an embryonic stem cell phenotype in lung adenocarcinoma. Clin. Cancer Res. 15, 7553–7561 (2009).
Schoenhals, M. et al. Embryonic stem cell markers expression in cancers. Biochem. Biophys. Res. Commun. 383, 157–162 (2009).
Al-Hajj, M., Wicha, M. S., Benito-Hernandez, A., Morrison, S. J. & Clarke, M. F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl Acad. Sci. USA 100, 3983–3988 (2003).
Collins, A. T., Berry, P. A., Hyde, C., Stower, M. J. & Maitland, N. J. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 65, 10946–10951 (2005).
Goldstein, A. S., Stoyanova, T. & Witte, O. Primitive origins of prostate cancer: in vivo evidence for prostate-regenerating cells and prostate cancer-initiating cells. Mol. Oncol. 4, 385–481 (2010).
Singh, S. K. et al. Identification of a cancer stem cell in human brain tumors. Neurosurgery 53, 487–488 (2003).
O'Brien, C. A., Pollett, A., Gallinger, S. & Dick, J. E. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 445, 106–110 (2007).
Clevers, H. The cancer stem cell: premises, promises and challenges. Nat. Med. 17, 313–319 (2011).
Visvader, J. E. & Lindeman, G. J. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat. Rev. Cancer 8, 755–768 (2008).
Patrawala, L., Calhoun-Davis, T., Schneider-Broussard, R. & Tang, D. G. Hierarchical organization of prostate cancer cells in xenograft tumors: the CD44+α2β1+ cell population is enriched in tumor-initiating cells. Cancer Res. 67, 6796–6805 (2007).
Frank, N. Y., Schatton, T. & Frank, M. H. The therapeutic promise of the cancer stem cell concept. J. Clin. Invest. 120, 41–50 (2010).
Alison, M. R., Lim, S. M. & Nicholson, L. J. Cancer stem cells: problems for therapy? J. Pathol. 223, 148–162 (2011).
Zhou, B. B. et al. Tumour-initiating cells: challenges and opportunities for anticancer drug discovery. Nat. Rev. Drug Discov. 8, 806–823 (2009).
Maitland, N. J., Frame, F. M., Polson, E. S., Lewis, J. L. & Collins, A. T. Prostate cancer stem cells: do they have a basal or luminal phenotype? Horm. Cancer 2, 47–61 (2011).
Oldridge, E. E., Pellacani, D., Collins, A. T. & Maitland, N. J. Prostate cancer stem cells: are they androgen-responsive? Mol. Cell Endocrinol. 360, 14–24 (2011).
van Leenders, G. J., Aalders, T. W., Hulsbergen-van de Kaa, C. A., Ruiter, D. J. & Schalken, J. A. Expression of basal cell keratins in human prostate cancer metastases and cell lines. J. Pathol. 195, 563–570 (2001).
Rizzo, S., Attard, G. & Hudson, D. L. Prostate epithelial stem cells. Cell Prolif. 38, 363–374 (2005).
Gil-Diez de Medina, S. et al. Modulation of cytokeratin subtype, EGF receptor, and androgen receptor expression during progression of prostate cancer. Hum. Pathol. 29, 1005–1012 (1998).
Hoey, T. et al. DLL4 blockade inhibits tumor growth and reduces tumor-initiating cell frequency. Cell Stem Cell 5, 168–177 (2009).
Gupta, P. B. et al. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell 138, 645–659 (2009).
Mueller, M. T. et al. Combined targeted treatment to eliminate tumorigenic cancer stem cells in human pancreatic cancer. Gastroenterology 137, 1102–1113 (2009).
Ma, S., Lee, T. K., Zheng, B. J., Chan, K. W. & Guan, X. Y. CD133+ HCC cancer stem cells confer chemoresistance by preferential expression of the Akt/PKB survival pathway. Oncogene 27, 1749–1758 (2008).
Levina, V., Marrangoni, A. M., DeMarco, R., Gorelik, E. & Lokshin, A. E. Drug-selected human lung cancer stem cells: cytokine network, tumorigenic and metastatic properties. PLoS ONE 3, e3077 (2008).
Dylla, S. J. et al. Colorectal cancer stem cells are enriched in xenogeneic tumors following chemotherapy. PLoS ONE 3, e2428 (2008).
Pierce, G. B. & Speers, W. C. Tumors as caricatures of the process of tissue renewal: prospects for therapy by directing differentiation. Cancer Res. 48, 1996–2004 (1988).
Wijaya, L., Agustina, D., Lizandi, A. O., Kartawinata, M. M. & Sandra, F. Reversing breast cancer stem cell into breast somatic stem cell. Curr. Pharm. Biotechnol. 12, 189–195 (2011).
Petrigliano, F. A. et al. Targeting of prostate cancer cells by a cytotoxic lentiviral vector containing a prostate stem cell antigen (PSCA) promoter. Prostate 69, 1422–1434 (2009).
El-Alfy, M., Pelletier, G., Hermo, L. S. & Labrie, F. Unique features of the basal cells of human prostate epithelium. Microsc. Res. Tech. 51, 436–446 (2000).
Humphrey, P. A. Diagnosis of adenocarcinoma in prostate needle biopsy tissue. J. Clin. Pathol. 60, 35–42 (2007).
Nagle, R. B. et al. Cytokeratin characterization of human prostatic carcinoma and its derived cell lines. Cancer Res. 47, 281–286 (1987).
Kuramoto, K. et al. The impact of low-dose busulfan on clonal dynamics in nonhuman primates. Blood 104, 1273–1280 (2004).
Calcagno, A. M. et al. Prolonged drug selection of breast cancer cells and enrichment of cancer stem cell characteristics. J. Natl Cancer Inst. 102, 1637–1652 (2010).
Maitland, N. J. & Collins, A. T. Cancer stem cells—a therapeutic target? Curr. Opin. Mol. Ther. 12, 662–673 (2010).
Sell, S. Stem cell origin of cancer and differentiation therapy. Crit. Rev. Oncol. Hematol. 51, 1–28 (2004).
Huang, M. E. et al. Use of all-trans retinoic acid in the treatment of acute promyelocytic leukemia. Blood 72, 567–572 (1988).
Rowley, J. D., Golomb, H. M. & Dougherty, C. 15/17 translocation, a consistent chromosomal change in acute promyelocytic leukaemia. Lancet 1, 549–550 (1977).
de Thé, H. et al. The PML-RAR α fusion mRNA generated by the t(15;17) translocation in acute promyelocytic leukemia encodes a functionally altered RAR. Cell 66, 675–684 (1991).
Grignani, F. et al. The acute promyelocytic leukemia-specific PML-RAR α fusion protein inhibits differentiation and promotes survival of myeloid precursor cells. Cell 74, 423–431 (1993).
Sanz, M. A. et al. Management of acute promyelocytic leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood 113, 1875–1891 (2009).
Nowak, D., Stewart, D. & Koeffler, H. P. Differentiation therapy of leukemia: 3 decades of development. Blood 113, 3655–3665 (2009).
Debruyne, F. J. et al. Liarozole—a novel treatment approach for advanced prostate cancer: results of a large randomized trial versus cyproterone acetate. Liarozole Study Group. Urology 52, 72–81 (1998).
Denis, L., Debruyne, F., De Porre, P. & Bruynseels, J. Early clinical experience with liarozole (Liazal) in patients with progressive prostate cancer. Eur. J. Cancer 34, 469–475 (1998).
Swami, S., Krishnan, A. V. & Feldman, D. Vitamin D metabolism and action in the prostate: implications for health and disease. Mol. Cell Endocrinol. 347, 61–69 (2011).
Pasquali, D., Rossi, V., Bellastella, G., Bellastella, A. & Sinisi, A. A. Natural and synthetic retinoids in prostate cancer. Curr. Pharm. Des. 12, 1923–1929 (2006).
Kubota, T. et al. Ligand for peroxisome proliferator-activated receptor gamma (troglitazone) has potent antitumor effect against human prostate cancer both in vitro and in vivo. Cancer Res. 58, 3344–3352 (1998).
Samid, D., Shack, S. & Myers, C. E. Selective growth arrest and phenotypic reversion of prostate cancer cells in vitro by nontoxic pharmacological concentrations of phenylacetate. J. Clin. Invest. 91, 2288–2295 (1993).
Floryk, D. & Huberman, E. Mycophenolic acid-induced replication arrest, differentiation markers and cell death of androgen-independent prostate cancer cells DU145. Cancer Lett. 231, 20–29 (2006).
Mueller, E. et al. Effects of ligand activation of peroxisome proliferator-activated receptor γ in human prostate cancer. Proc. Natl Acad. Sci. USA 97, 10990–10995 (2000).
Leibowitz, S. B. & Kantoff, P. W. Differentiating agents and the treatment of prostate cancer: vitamin D3 and peroxisome proliferator-activated receptor gamma ligands. Semin. Oncol. 30, 698–708 (2003).
Shiau, C. W. et al. Thiazolidenediones mediate apoptosis in prostate cancer cells in part through inhibition of Bcl-xL/Bcl-2 functions independently of PPARγ. Cancer Res. 65, 1561–1569 (2005).
Akinyeke, T. O. & Stewart, L. V. Troglitazone suppresses c-Myc levels in human prostate cancer cells via a PPARgamma-independent mechanism. Cancer Biol. Ther. 11, 1046–1058 (2011).
Crowe, D. L., Kim, R. & Chandraratna, R. A. Retinoic acid differentially regulates cancer cell proliferation via dose-dependent modulation of the mitogen-activated protein kinase pathway. Mol. Cancer Res. 1, 532–540 (2003).
Hurt, E. M., Kawasaki, B. T., Klarmann, G. J., Thomas, S. B. & Farrar, W. L. CD44+ CD24− prostate cells are early cancer progenitor/stem cells that provide a model for patients with poor prognosis. Br. J. Cancer 98, 756–765 (2008).
Esquenet, M., Swinnen, J. V., Heyns, W. & Verhoeven, G. Control of LNCaP proliferation and differentiation: actions and interactions of androgens, 1α,25-dihydroxycholecalciferol, all-trans retinoic acid, 9-cis retinoic acid, and phenylacetate. Prostate 28, 182–194 (1996).
Gleave, M. E. et al. Butyrate analogue, isobutyramide, inhibits tumor growth and time to androgen-independent progression in the human prostate LNCaP tumor model. J. Cell Biochem. 69, 271–281 (1998).
Hisatake, J. I. et al. Down-regulation of prostate-specific antigen expression by ligands for peroxisome proliferator-activated receptor gamma in human prostate cancer. Cancer Res. 60, 5494–5498 (2000).
Hedlund, T. E., Moffatt, K. A., Uskokovic, M. R. & Miller, G. J. Three synthetic vitamin D analogues induce prostate-specific acid phosphatase and prostate-specific antigen while inhibiting the growth of human prostate cancer cells in a vitamin D receptor-dependent fashion. Clin. Cancer Res. 3, 1331–1338 (1997).
Floryk, D. & Thompson, T. C. Perifosine induces differentiation and cell death in prostate cancer cells. Cancer Lett. 266, 216–226 (2008).
Woo, T. C., Choo, R., Jamieson, M., Chander, S. & Vieth, R. Pilot study: potential role of vitamin D (Cholecalciferol) in patients with PSA relapse after definitive therapy. Nutr. Cancer 51, 32–36 (2005).
Ottinger, S. et al. Targeting of pancreatic and prostate cancer stem cell characteristics by Crambe crambe marine sponge extract. Int. J. Cancer 130, 1671–1681 (2011).
Hellsten, R., Johansson, M., Dahlman, A., Sterner, O. & Bjartell, A. Galiellalactone inhibits stem cell-like ALDH-positive prostate cancer cells. PLoS ONE 6, e22118 (2011).
Kallifatidis, G. et al. Sulforaphane increases drug-mediated cytotoxicity toward cancer stem-like cells of pancreas and prostate. Mol. Ther. 19, 188–195 (2011).
Marian, C. O., Wright, W. E. & Shay, J. W. The effects of telomerase inhibition on prostate tumor-initiating cells. Int. J. Cancer 127, 321–331 (2010).
Dubrovska, A. et al. The role of PTEN/Akt/PI3K signaling in the maintenance and viability of prostate cancer stem-like cell populations. Proc. Natl Acad. Sci. USA 106, 268–273 (2009).
Charrad, R. S. et al. Ligation of the CD44 adhesion molecule reverses blockage of differentiation in human acute myeloid leukemia. Nat. Med. 5, 669–676 (1999).
Dubrovska, A. et al. Combination therapy targeting both tumor-initiating and differentiated cell populations in prostate carcinoma. Clin. Cancer Res. 16, 5692–5702 (2010).
Liu, C. et al. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat. Med. 17, 211–215 (2011).
Luk, S. U. et al. γ-tocotrienol as an effective agent in targeting prostate cancer stem cell-like population. Int. J. Cancer 128, 2182–2191 (2011).
Klein, E. A. et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 306, 1549–1556 (2011).
Gysin, R., Azzi, A. & Visarius, T. γ-Tocopherol inhibits human cancer cell cycle progression and cell proliferation by down-regulation of cyclins. FASEB J. 16, 1952–1954 (2002).
Venkateswaran, V., Fleshner, N. E. & Klotz, L. H. Modulation of cell proliferation and cell cycle regulators by vitamin E in human prostate carcinoma cell lines. J. Urol. 168, 1578–1582 (2002).
Packer, J. E., Slater, T. F. & Willson, R. L. Direct observation of a free radical interaction between vitamin E and vitamin C. Nature 278, 737–738 (1979).
Mimeault, M. & Batra, S. K. Animal models relevant to human prostate carcinogenesis underlining the critical implication of prostatic stem/progenitor cells. Biochim. Biophys. Acta 1816, 25–37 (2011).
Richardson, G. D. et al. CD133, a novel marker for human prostatic epithelial stem cells. J. Cell Sci. 117, 3539–3545 (2004).
Lawson, D. A. et al. Basal epithelial stem cells are efficient targets for prostate cancer initiation. Proc. Natl Acad. Sci. USA 107, 2610–2615 (2010).
Birnie, R. et al. Gene expression profiling of human prostate cancer stem cells reveals a pro-inflammatory phenotype and the importance of extracellular matrix interactions. Genome Biol. 9, R83 (2008).
Shepherd, C. J. et al. Expression profiling of CD133+ and CD133− epithelial cells from human prostate. Prostate 68, 1007–1024 (2008).
Maund, S. L. et al. Interleukin-1α mediates the antiproliferative effects of 1,25-dihydroxyvitamin D3 in prostate progenitor/stem cells. Cancer Res. 71, 5276–5286 (2011).
Richards, J. et al. Interactions of abiraterone, eplerenone and prednisolone with wild-type and mutant androgen receptor: a rationale for increasing abiraterone exposure or combining with MDV3100. Cancer Res. 72, 2176–2182 (2012).
Stein, M. N., Goodin, S. & Dipaola, R. S. Abiraterone in prostate cancer: a new angle to an old problem. Clin. Cancer Res. 18, 1848–1854 (2012).
Parker, C. et al. Overall survival benefit of radium-223 chloride (AlpharadinTM) in the treatment of patients with symptomatic bone metastases in castration-resistant prostate cancer (CRPC): a phase III randomized trial (ALSYMPCA) [abstract]. Eur. J. Cancer 47 (Suppl. 2), 3 (2011).
de Bono, J. S. et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 376, 1147–1154 (2010).
Scher, H. I. et al. Effect of MDV3100, an androgen receptor signaling inhibitor (ARSI), on overall survival in patients with prostate cancer postdocetaxel: results from the phase III AFFIRM study [abstract]. J. Clin. Oncol. 30 (Suppl. 5), LBA1 (2012).
Acknowledgements
We would like to thank Dr Fiona Frame and Dr Euan Polson for their critical reading of the manuscript before submission. We would also like to acknowledge our funding from the EU Marie Curie Network: Prostate Research Organizations–Network of Early Stage Training (J. K. Rane), The Freemasons' Grand Charity (D. Pellacani) and Yorkshire Cancer Research (Project grant Y256 and Program grant) (D. Pellacani, N. J. Maitland).
Author information
Authors and Affiliations
Contributions
J. K. Rane and D. Pellacani researched the data for and wrote the article. All authors contributed substantially to the discussion of the article content and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Rane, J., Pellacani, D. & Maitland, N. Advanced prostate cancer—a case for adjuvant differentiation therapy. Nat Rev Urol 9, 595–602 (2012). https://doi.org/10.1038/nrurol.2012.157
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrurol.2012.157
This article is cited by
-
The immunosuppressive cytokine interleukin-4 increases the clonogenic potential of prostate stem-like cells by activation of STAT6 signalling
Oncogenesis (2017)
-
Context dependent regulatory patterns of the androgen receptor and androgen receptor target genes
BMC Cancer (2016)
-
Loss of SLCO1B3 drives taxane resistance in prostate cancer
British Journal of Cancer (2016)
-
DNA hypermethylation in prostate cancer is a consequence of aberrant epithelial differentiation and hyperproliferation
Cell Death & Differentiation (2014)