Abstract
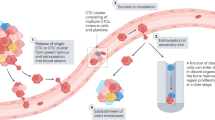
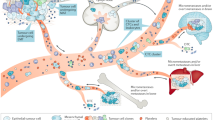
The loss of single cells from a tumour cell cluster marks an early event in the metastatic process of cancer progression. Although the metastatic cascade in prostate cancer is yet to be fully understood, monitoring circulating tumour cells (CTCs) and quantifying the load of tumour cell dissemination is currently being implemented into routine clinical practice for diagnosing minimal residual disease (MRD), estimating prognosis and monitoring treatment success. Current methods for enrichment of CTCs or disseminated tumour cells (DTCs) and detection of MRD rely on the expression of specific marker genes or proteins that might be altered during the process of tumour cell dissemination, therefore disrupting tumour cell detection. The tumour origin and malignant potential for metastasis of marker-positive cells is not yet clear. Some studies have demonstrated the potential of CTCs or DTCs as prognostic or predictive markers, leading to the increasing implementation of CTC measurement as an end point in clinical trials.
Key Points
-
Systemic shedding of tumour cells marks an early event in tumorigenesis and is a prerequisite for distant recurrence after initial curative therapy
-
Evidence of minimal residual disease (MRD) is a negative prognosticator for prostate cancer
-
Current methods for the enrichment and detection of MRD rely on the expression of specific marker genes or proteins that might be altered during the process of tumour cell dissemination
-
Tumour origin and malignant potential for metastasis of marker-positive cells is not yet clear
-
Circulating and disseminated tumour cells have been reported as prognostic or predictive markers in prostate cancer leading to their increasing implementation as additional end points in clinical trials
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Center, M. M. et al. International variation in prostate cancer incidence and mortality rates. Eur. Urol. 61, 1079–1092 (2012).
Bill-Axelson, A. et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N. Engl. J. Med. 364, 1708–1717 (2011).
Yossepowitch, O. et al. The natural history of noncastrate metastatic prostate cancer after radical prostatectomy. Eur. Urol. 51, 940–947 (2007).
Loeb, S. et al. Can we stop prostate specific antigen testing 10 years after radical prostatectomy? J. Urol. 186, 500–505 (2011).
Butler, T. P. & Gullino, P. M. Quantitation of cell shedding into efferent blood of mammary adenocarcinoma. Cancer Res. 35, 512–516 (1975).
Ghossein, R. A. et al. Detection of circulating tumor cells in patients with localized and metastatic prostatic carcinoma: clinical implications. J. Clin. Oncol. 13, 1195–1200 (1995).
Seiden, M. V. et al. Detection of circulating tumor cells in men with localized prostate cancer. J. Clin. Oncol. 12, 2634–2639 (1994).
Sugarbaker, E. V., Cohen, A. M. & Ketcham, A. S. Facilitated metastatic distribution of the Walker 256 tumor in Sprague-Dawley rats with hydrocortisone and-or cyclophosphamide. J. Surg. Oncol. 2, 277–289 (1970).
Kollermann, J. et al. Prognostic significance of disseminated tumor cells in the bone marrow of prostate cancer patients treated with neoadjuvant hormone treatment. J. Clin. Oncol. 26, 4928–4933 (2008).
Cheng, L. et al. Risk of prostate carcinoma death in patients with lymph node metastasis. Cancer 91, 66–73 (2001).
Hull, G. W. et al. Cancer control with radical prostatectomy alone in 1,000 consecutive patients. J. Urol. 167, 528–534 (2002).
Yates, D. R. et al. Quantitative RT-PCR analysis of PSA and prostate-specific membrane antigen mRNA to detect circulating tumor cells improves recurrence-free survival nomogram prediction after radical prostatectomy. Prostate http://dx.doi.org/10.1002/pros.22488.
Schmidt, H. et al. Asynchronous growth of prostate cancer is reflected by circulating tumor cells delivered from distinct, even small foci, harboring loss of heterozygosity of the PTEN gene. Cancer Res. 66, 8959–8965 (2006).
Hawksworth, D. et al. Overexpression of C-MYC oncogene in prostate cancer predicts biochemical recurrence. Prostate Cancer Prostatic Dis. 13, 311–315 (2010).
Barwick, B. G. et al. Prostate cancer genes associated with TMPRSS2-ERG gene fusion and prognostic of biochemical recurrence in multiple cohorts. Br. J. Cancer 102, 570–576 (2010).
Schlomm, T. et al. Clinical significance of p53 alterations in surgically treated prostate cancers. Mod. Pathol. 21, 1371–1378 (2008).
Pienta, K. J. & Loberg, R. The “emigration, migration, and immigration” of prostate cancer. Clin. Prostate Cancer 4, 24–30 (2005).
Mol, A. J., Geldof, A. A., Meijer, G. A., van der Poel, H. G. & van Moorselaar, R. J. New experimental markers for early detection of high-risk prostate cancer: role of cell-cell adhesion and cell migration. J. Cancer Res. Clin. Oncol. 133, 687–695 (2007).
Gravdal, K., Halvorsen, O. J., Haukaas, S. A. & Akslen, L. A. A switch from E-cadherin to N-cadherin expression indicates epithelial to mesenchymal transition and is of strong and independent importance for the progress of prostate cancer. Clin. Cancer Res. 13, 7003–7011 (2007).
Arya, M. et al. The metastatic cascade in prostate cancer. Surg. Oncol. 15, 117–128 (2006).
Kumano, M. et al. Expression of urokinase-type plasminogen activator system in prostate cancer: correlation with clinicopathological outcomes in patients undergoing radical prostatectomy. Urol. Oncol. 27, 180–186 (2009).
Trudel, D., Fradet, Y., Meyer, F., Harel, F. & Tetu, B. Significance of MMP-2 expression in prostate cancer: an immunohistochemical study. Cancer Res. 63, 8511–8515 (2003).
Sanda, M. G. et al. Molecular characterization of defective antigen processing in human prostate cancer. J. Natl Cancer Inst. 87, 280–285 (1995).
Taichman, R. S. et al. Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res. 62, 1832–1837 (2002).
Cooper, C. R. et al. Preferential adhesion of prostate cancer cells to bone is mediated by binding to bone marrow endothelial cells as compared to extracellular matrix components in vitro. Clin. Cancer Res. 6, 4839–4847 (2000).
Glinsky, V. V. et al. The role of Thomsen-Friedenreich antigen in adhesion of human breast and prostate cancer cells to the endothelium. Cancer Res. 61, 4851–4857 (2001).
Barrett, J. M., Mangold, K. A., Jilling, T. & Kaul, K. L. Bi-directional interactions of prostate cancer cells and bone marrow endothelial cells in three-dimensional culture. Prostate 64, 75–82 (2005).
Jin, J. K., Dayyani, F. & Gallick, G. E. Steps in prostate cancer progression that lead to bone metastasis. Int. J. Cancer 128, 2545–2561 (2011).
Yates, C. C., Shepard, C. R., Stolz, D. B. & Wells, A. Co-culturing human prostate carcinoma cells with hepatocytes leads to increased expression of E-cadherin. Br. J. Cancer 96, 1246–1252 (2007).
Saha, B. et al. Overexpression of E-cadherin and beta-catenin proteins in metastatic prostate cancer cells in bone. Prostate 68, 78–84 (2008).
Pantel, K. & Brakenhoff, R. H. Dissecting the metastatic cascade. Nat. Rev. Cancer 4, 448–456 (2004).
Miller, M. C., Doyle, G. V. & Terstappen, L. W. Significance of circulating tumor cells detected by the CellSearch System in patients with metastatic breast colorectal and prostate cancer. J. Oncol. 2010, 617421 (2010).
Schmitt, M. & Foekens, J. A. Circulating tumor cells in blood of primary breast cancer patients assessed by a novel RT-PCR test kit and comparison with status of bone marrow-disseminated tumor cells. Breast Cancer Res. 11, 109 (2009).
Stapleton, A. M. et al. Primary human prostate cancer cells harboring p53 mutations are clonally expanded in metastases. Clin. Cancer Res. 3, 1389–1397 (1997).
Paris, P. L. et al. Functional phenotyping and genotyping of circulating tumor cells from patients with castration resistant prostate cancer. Cancer Lett. 277, 164–173 (2009).
Attard, G. et al. Characterization of ERG, AR and PTEN gene status in circulating tumor cells from patients with castration-resistant prostate cancer. Cancer Res. 69, 2912–2918 (2009).
Oberneder, R. et al. Immunocytochemical detection and phenotypic characterization of micrometastatic tumour cells in bone marrow of patients with prostate cancer. Urol. Res. 22, 3–8 (1994).
Alix-Panabieres, C. et al. Detection and characterization of putative metastatic precursor cells in cancer patients. Clin. Chem. 53, 537–539 (2007).
Jenkins, R. B., Qian, J., Lieber, M. M. & Bostwick, D. G. Detection of c-myc oncogene amplification and chromosomal anomalies in metastatic prostatic carcinoma by fluorescence in situ hybridization. Cancer Res. 57, 524–531 (1997).
Schilling, D. et al. Quantification of tumor cell burden by analysis of single cell lymph node disaggregates in metastatic prostate cancer. Prostate 70, 1110–1118 (2010).
de la Taille, A. et al. Detection of prostate-specific membrane antigen expressing cells in blood obtained from renal cancer patients: a potential biomarker of vascular invasion. Cancer Detect. Prev. 24, 579–588 (2000).
Racila, E. et al. Detection and characterization of carcinoma cells in the blood. Proc. Natl Acad. Sci. USA 95, 4589–4594 (1998).
Zieglschmid, V., Hollmann, C. & Bocher, O. Detection of disseminated tumor cells in peripheral blood. Crit. Rev. Clin. Lab. Sci. 42, 155–196 (2005).
Nagrath, S. et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 450, 1235–1239 (2007).
Vona, G. et al. Isolation by size of epithelial tumor cells: a new method for the immunomorphological and molecular characterization of circulatingtumor cells. Am. J. Pathol. 156, 57–63 (2000).
Gertler, R. et al. Detection of circulating tumor cells in blood using an optimized density gradient centrifugation. Recent Results Cancer Res. 162, 149–155 (2003).
Gleghorn, J. P. et al. Capture of circulating tumor cells from whole blood of prostate cancer patients using geometrically enhanced differential immunocapture (GEDI) and a prostate-specific antibody. Lab Chip 10, 27–29 (2010).
Antolovic, D. et al. Heterogeneous detection of circulating tumor cells in patients with colorectal cancer by immunomagnetic enrichment using different EpCAM-specific antibodies. BMC Biotechnol. 10, 35 (2010).
Allan, A. L. & Keeney, M. Circulating tumor cell analysis: technical and statistical considerations for application to the clinic. J. Oncol. 2010, 426218 (2010).
Aktas, B. et al. Stem cell and epithelial-mesenchymal transition markers are frequently overexpressed in circulating tumor cells of metastatic breast cancer patients. Breast Cancer Res. 11, R46 (2009).
He, W. et al. Quantitation of circulating tumor cells in blood samples from ovarian and prostate cancer patients using tumor-specific fluorescent ligands. Int. J. Cancer 123, 1968–1973 (2008).
Shaffer, D. R. et al. Circulating tumor cell analysis in patients with progressive castration-resistant prostate cancer. Clin. Cancer Res. 13, 2023–2029 (2007).
Ghossein, R. A., Carusone, L. & Bhattacharya, S. Review: polymerase chain reaction detection of micrometastases and circulating tumor cells: application to melanoma, prostate, and thyroid carcinomas. Diagn. Mol. Pathol. 8, 165–175 (1999).
Helo, P. et al. Circulating prostate tumor cells detected by reverse transcription-PCR in men with localized or castration-refractory prostate cancer: concordance with CellSearch assay and association with bone metastases and with survival. Clin. Chem. 55, 765–773 (2009).
Andreopoulou, E. et al. Comparison of assay methods for detection of circulating tumor cells in metastatic breast cancer: AdnaGen AdnaTest BreastCancer Select/Detect versus Veridex CellSearch system. Int. J. Cancer 130, 1590–1597 (2012).
Todenhoefer, T. et al. PCR-based detection of circulating tumor cells in prostate cancer—preliminary experience. Eur. Urol. Suppl. 11, e429 (2012).
Berg, A. et al. Disseminated tumor cells in bone marrow following definitive radiotherapy for intermediate or high-risk prostate cancer. Prostate 68, 1607–1614 (2008).
Ikeda, S. et al. Combined immunohistochemistry of beta-catenin, cytokeratin 7, and cytokeratin 20 is useful in discriminating primary lung adenocarcinomas from metastatic colorectal cancer. BMC Cancer 6, 31 (2006).
Woelfle, U., Sauter, G., Santjer, S., Brakenhoff, R. & Pantel, K. Down-regulated expression of cytokeratin 18 promotes progression of human breast cancer. Clin. Cancer Res. 10, 2670–2674 (2004).
Fehm, T. et al. A concept for the standardized detection of disseminated tumor cells in bone marrow from patients with primary breast cancer and its clinical implementation. Cancer 107, 885–892 (2006).
Panteleakou, Z. et al. Detection of circulating tumor cells in prostate cancer patients: methodological pitfalls and clinical relevance. Mol. Med. 15, 101–114 (2009).
Trzpis, M., McLaughlin, P. M., de Leij, L. M. & Harmsen, M. C. Epithelial cell adhesion molecule: more than a carcinoma marker and adhesion molecule. Am. J. Pathol. 171, 386–395 (2007).
Benko, G., Spajic, B., Kruslin, B. & Tomas, D. Impact of the EpCAM expression on biochemical recurrence-free survival in clinically localized prostate cancer. Urol. Oncol. http://dx.doi.org/10.1016/j.urolonc.2011.03.007.
McIntyre, I. G. et al. The molecular staging of prostate cancer. BJU Int. 94, 1217–1220 (2004).
Katz, A. E. et al. Molecular staging of prostate cancer with the use of an enhanced reverse transcriptase-PCR assay. Urology 43, 765–775 (1994).
Ghossein, R. A., Carusone, L. & Bhattacharya, S. Molecular detection of micrometastases and circulating tumor cells in melanoma prostatic and breast carcinomas. In Vivo 14, 237–250 (2000).
Corey, E. & Corey, M. J. Detection of disseminated prostate cells by reverse transcription-polymerase chain reaction (RT-PCR): technical and clinical aspects. Int. J. Cancer 77, 655–673 (1998).
Zippelius, A., Lutterbuse, R., Riethmuller, G. & Pantel, K. Analytical variables of reverse transcription-polymerase chain reaction-based detection of disseminated prostate cancer cells. Clin. Cancer Res. 6, 2741–2750 (2000).
Berois, N. et al. Molecular detection of cancer cells in bone marrow and peripheral blood of patients with operable breast cancer. Comparison of CK19, MUC1 and CEA using RT-PCR. Eur. J. Cancer 36, 717–723 (2000).
Kusuda, Y., Miyake, H., Kurahashi, T. & Fujisawa, M. Assessment of optimal target genes for detecting micrometastases in pelvic lymph nodes in patients with prostate cancer undergoing radical prostatectomy by real-time reverse transcriptase-polymerase chain reaction. Urol. Oncol. (2011).
Devriese, L. A. et al. Circulating tumor cell detection in advanced non-small cell lung cancer patients by multi-marker QPCR analysis. Lung Cancer 75, 242–247 (2011).
Chen, Y. et al. Detection of cytokeratin 19, human mammaglobin, and carcinoembryonic antigen-positive circulating tumor cells by three-marker reverse transcription-PCR assay and its relation to clinical outcome in early breast cancer. Int. J. Biol. Markers 25, 59–68 (2010).
Henke, W. et al. Increased analytical sensitivity of RT-PCR of PSA mRNA decreases diagnostic specificity of detection of prostatic cells in blood. Int. J. Cancer 70, 52–56 (1997).
Lintula, S. & Stenman, U. H. The expression of prostate-specific membrane antigen in peripheral blood leukocytes. J. Urol. 157, 1969–1972 (1997).
Lambrechts, A. C. et al. Comparison of immunocytochemistry, reverse transcriptase polymerase chain reaction, and nucleic acid sequence-based amplification for the detection of circulating breast cancer cells. Breast Cancer Res. Treat. 56, 219–231 (1999).
Balducci, E. et al. A new nested primer pair improves the specificity of CK-19 mRNA detection by RT-PCR in occult breast cancer cells. Int. J. Biol. Markers 20, 28–33 (2005).
Corey, E. et al. Detection of circulating prostate cells by reverse transcriptase-polymerase chain reaction of human glandular kallikrein (hK2) and prostate-specific antigen (PSA) messages. Urology 50, 184–188 (1997).
Mukherjee, S. et al. Identification of EpCAM as a molecular target of prostate cancer stroma. Am. J. Pathol. 175, 2277–2287 (2009).
Bussemakers, M. J. et al. DD3: a new prostate-specific gene, highly overexpressed in prostate cancer. Cancer Res. 59, 5975–5979 (1999).
van Gils, M. P. et al. The time-resolved fluorescence-based PCA3 test on urinary sediments after digital rectal examination; a Dutch multicenter validation of the diagnostic performance. Clin. Cancer Res. 13, 939–943 (2007).
Lin, H. K. et al. Portable filter-based microdevice for detection and characterization of circulating tumor cells. Clin. Cancer Res. 16, 5011–5018 (2010).
Danila, D. C., Fleisher, M. & Scher, H. I. Circulating tumor cells as biomarkers in prostate cancer. Clin. Cancer Res. 17, 3903–3912 (2011).
Riethdorf, S. et al. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the CellSearch system. Clin. Cancer Res. 13, 920–928 (2007).
Bonnomet, A. et al. Epithelial-to-mesenchymal transitions and circulating tumor cells. J. Mammary Gland Biol. Neoplasia 15, 261–273 (2010).
Armstrong, A. J. et al. Circulating tumor cells from patients with advanced prostate and breast cancer display both epithelial and mesenchymal markers. Mol. Cancer Res. 9, 997–1007 (2011).
Wang, L. et al. Flow cytometric analysis of CK19 expression in the peripheral blood of breast carcinoma patients: relevance for circulating tumor cell detection. J. Exp. Clin. Cancer Res. 28, 57 (2009).
Riethdorf, S., Wikman, H. & Pantel, K. Review: Biological relevance of disseminated tumor cells in cancer patients. Int. J. Cancer 123, 1991–2006 (2008).
Morgan, T. M. et al. Disseminated tumor cells in prostate cancer patients after radical prostatectomy and without evidence of disease predicts biochemical recurrence. Clin. Cancer Res. 15, 677–683 (2009).
Fehm, T. et al. HER2 status of circulating tumor cells in patients with metastatic breast cancer: a prospective, multicenter trial. Breast Cancer Res. Treat. 124, 403–412 (2010).
Stott, S. L. et al. Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proc. Natl Acad. Sci. USA 107, 18392–18397 (2010).
Tomlins, S. A. et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 310, 644–648 (2005).
Danila, D. C. et al. TMPRSS2-ERG status in circulating tumor cells as a predictive biomarker of sensitivity in castration-resistant prostate cancer patients treated with abiraterone acetate. Eur. Urol. 60, 897–904 (2011).
Moul, J. W. et al. Immunohistologic detection of prostate cancer pelvic lymph node micrometastases: correlation to preoperative serum prostate-specific antigen. Urology 43, 68–73 (1994).
Gomella, L. G., White, J. L., McCue, P. A., Byrne, D. S. & Mulholland, S. G. Screening for occult nodal metastasis in localized carcinoma of the prostate. J. Urol. 149, 776–778 (1993).
Martinez-Pineiro, L. et al. Molecular staging of prostatic cancer with RT-PCR assay for prostate-specific antigen in peripheral blood and lymph nodes: comparison with standard histological staging and immunohistochemical assessment of occult regional lymph node metastases. Eur. Urol. 43, 342–350 (2003).
Wawroschek, F. et al. The influence of serial sections, immunohistochemistry, and extension of pelvic lymph node dissection on the lymph node status in clinically localized prostate cancer. Eur. Urol. 43, 132–136 (2003).
Fukuda, M. et al. Detection of sentinel node micrometastasis by step section and immunohistochemistry in patients with prostate cancer. J. Urol. 177, 1313–1317 (2007).
Schilling, D. et al. Prospective assessment of histological serial sectioning of pelvic lymph nodes in prostate cancer: a cost-benefit analysis. BJU Int. http://dx.doi.org/10.1111/j.1464-410X.2012.10928.x.
Epstein, J. I., Srigley, J., Grignon, D. & Humphrey, P. Recommendations for the reporting of prostate carcinoma: Association of Directors of Anatomic and Surgical Pathology. Am. J. Clin. Pathol. 129, 24–30 (2008).
Moreno, J. G. et al. Circulating tumor cells predict survival in patients with metastatic prostate cancer. Urology 65, 713–718 (2005).
Danila, D. C. et al. Circulating tumor cell number and prognosis in progressive castration-resistant prostate cancer. Clin. Cancer Res. 13, 7053–7058 (2007).
Scher, H. I. et al. Circulating tumour cells as prognostic markers in progressive, castration-resistant prostate cancer: a reanalysis of IMMC38 trial data. Lancet Oncol. 10, 233–239 (2009).
de Bono, J. S. et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin. Cancer Res. 14, 6302–6309 (2008).
Olmos, D. et al. Circulating tumour cell (CTC) counts as intermediate end points in castration-resistant prostate cancer (CRPC): a single-centre experience. Ann. Oncol. 20, 27–33 (2009).
Goodman, O. B. Jr et al. Circulating tumor cells as a predictive biomarker in patients with hormone-sensitive prostate cancer. Clin. Genitourin. Cancer 9, 31–38 (2011).
Chen, B. T. et al. Preliminary study of immunomagnetic quantification of circulating tumor cells in patients with advanced disease. Urology 65, 616–621 (2005).
Davis, J. W. et al. Circulating tumor cells in peripheral blood samples from patients with increased serum prostate specific antigen: initial results in early prostate cancer. J. Urol. 179, 2187–2191 (2008).
Darshan, M. S. et al. Taxane-induced blockade to nuclear accumulation of the androgen receptor predicts clinical responses in metastatic prostate cancer. Cancer Res. 71, 6019–6029 (2011).
Jiang, Y., Palma, J. F., Agus, D. B., Wang, Y. & Gross, M. E. Detection of androgen receptor mutations in circulating tumor cells in castration-resistant prostate cancer. Clin. Chem. 56, 1492–1495 (2010).
de Bono, J. S. et al. Abiraterone and increased survival in metastatic prostate cancer. N. Engl. J. Med. 364, 1995–2005 (2011).
Scher, H. I. et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1–2 study. Lancet 375, 1437–1446 (2010).
Weckermann, D. et al. Disseminated cytokeratin positive tumor cells in the bone marrow of patients with prostate cancer: detection and prognostic value. J. Urol. 166, 699–703 (2001).
Weckermann, D., Wawroschek, F., Krawczak, G., Haude, K. H. & Harzmann, R. Does the immunocytochemical detection of epithelial cells in bone marrow (micrometastasis) influence the time to biochemical relapse after radical prostatectomy? Urol. Res. 27, 285–290 (1999).
Berg, A. et al. Impact of disseminated tumor cells in bone marrow at diagnosis in patients with nonmetastatic prostate cancer treated by definitive radiotherapy. Int. J. Cancer 120, 1603–1609 (2007).
Weckermann, D. et al. Perioperative activation of disseminated tumor cells in bone marrow of patients with prostate cancer. J. Clin. Oncol. 27, 1549–1556 (2009).
Wood, D. P. Jr & Banerjee, M. Presence of circulating prostate cells in the bone marrow of patients undergoing radical prostatectomy is predictive of disease-free survival. J. Clin. Oncol. 15, 3451–3457 (1997).
Gao, C. L. et al. Detection of circulating prostate specific antigen expressing prostatic cells in the bone marrow of radical prostatectomy patients by sensitive reverse transcriptase polymerase chain reaction. J. Urol. 161, 1070–1076 (1999).
Mitsiades, C. S. et al. Molecular staging by RT-pCR analysis for PSA and PSMA in peripheral blood and bone marrow samples is an independent predictor of time to biochemical failure following radical prostatectomy for clinically localized prostate cancer. Clin. Exp. Metastasis 21, 495–505 (2004).
Pfitzenmaier, J. et al. The detection and isolation of viable prostate-specific antigen positive epithelial cells by enrichment: a comparison to standard prostate-specific antigen reverse transcriptase polymerase chain reaction and its clinical relevance in prostate cancer. Urol. Oncol. 25, 214–220 (2007).
Pagliarulo, V. et al. Detection of occult lymph node metastases in locally advanced node-negative prostate cancer. J. Clin. Oncol. 24, 2735–2742 (2006).
Fleischmann, A., Schobinger, S., Schumacher, M., Thalmann, G. N. & Studer, U. E. Survival in surgically treated, nodal positive prostate cancer patients is predicted by histopathological characteristics of the primary tumor and its lymph node metastases. Prostate 69, 352–362 (2009).
Ferrari, A. C. et al. Molecular load of pathologically occult metastases in pelvic lymph nodes is an independent prognostic marker of biochemical failure after localized prostate cancer treatment. J. Clin. Oncol. 24, 3081–3088 (2006).
Schostak, M. et al. Does the molecular staging in pelvic lymph nodes improve the detection of relevant prostate cancer metastases? An assessment after 6 years. BJU Int. 99, 1409–1414 (2007).
Miyake, H. et al. Quantitative detection of micrometastases in pelvic lymph nodes in patients with clinically localized prostate cancer by real-time reverse transcriptase-PCR. Clin. Cancer Res. 13, 1192–1197 (2007).
Shariat, S. F. et al. Detection of clinically significant, occult prostate cancer metastases in lymph nodes using a splice variant-specific rt-PCR assay for human glandular kallikrein. J. Clin. Oncol. 21, 1223–1231 (2003).
Shariat, S. F. et al. Comparison of immunohistochemistry with reverse transcription-PCR for the detection of micrometastatic prostate cancer in lymph nodes. Cancer Res. 63, 4662–4670 (2003).
Srigley, J. R. et al. Updated protocol for the examination of specimens from patients with carcinomas of the prostate gland. Arch. Pathol. Lab. Med. 130, 936–946 (2006).
Reid, A. H. et al. Significant and sustained antitumor activity in post-docetaxel, castration-resistant prostate cancer with the CYP17 inhibitor abiraterone acetate. J. Clin. Oncol. 28, 1489–1495 (2010).
Danila, D. C. et al. Phase II multicenter study of abiraterone acetate plus prednisone therapy in patients with docetaxel-treated castration-resistant prostate cancer. J. Clin. Oncol. 28, 1496–1501 (2010).
Wittekind, C. Diagnosis and staging of lymph node metastasis. Recent Results Cancer Res. 157, 20–28 (2000).
Konigsberg, R. et al. Detection of EpCAM positive and negative circulating tumor cells in metastatic breast cancer patients. Acta Oncol. 50, 700–710 (2011).
Deng, G. et al. Enrichment with anti-cytokeratin alone or combined with anti-EpCAM antibodies significantly increases the sensitivity for circulating tumor cell detection in metastatic breast cancer patients. Breast Cancer Res. 10, R69 (2008).
Martin, V. M. et al. Immunomagnetic enrichment of disseminated epithelial tumor cells from peripheral blood by MACS. Exp. Hematol. 26, 252–264 (1998).
Fehm, T. et al. ERalpha-status of disseminated tumour cells in bone marrow of primary breast cancer patients. Breast Cancer Res. 10, R76 (2008).
Kollermann, J. et al. Methylation-specific PCR for DNA-based detection of occult tumor cells in lymph nodes of prostate cancer patients. Eur. Urol. 44, 533–538 (2003).
Mayer, J. A. et al. FISH-based determination of HER2 status in circulating tumor cells isolated with the microfluidic CEE platform. Cancer Genet. 204, 589–595 (2011).
Bianco, F. J., Jr, Powell, I. J., Cher, M. L. & Wood, D. P. Jr. Presence of circulating prostate cancer cells in African American males adversely affects survival. Urol. Oncol. 7, 147–152 (2002).
Joung, J. Y. et al. Prostate stem cell antigen mRNA in peripheral blood as a potential predictor of biochemical recurrence in high-risk prostate cancer. J. Surg. Oncol. 101, 145–148 (2010).
Shariat, S. F. et al. Preoperative blood reverse transcriptase-PCR assays for prostate-specific antigen and human glandular kallikrein for prediction of prostate cancer progression after radical prostatectomy. Cancer Res. 62, 5974–5979 (2002).
Goodman, O. B. Jr et al. Circulating tumor cells in patients with castration-resistant prostate cancer baseline values and correlation with prognostic factors. Cancer Epidemiol. Biomarkers Prev. 18, 1904–1913 (2009).
Okegawa, T., Nutahara, K. & Higashihara, E. Prognostic significance of circulating tumor cells in patients with hormone refractory prostate cancer. J. Urol. 181, 1091–1097 (2009).
Coumans, F. A., Doggen, C. J., Attard, G., de Bono, J. S. & Terstappen, L. W. All circulating EpCAM+CK+CD45- objects predict overall survival in castration-resistant prostate cancer. Ann. Oncol. 21, 1851–1857 (2010).
Strijbos, M. H. et al. Circulating endothelial cells, circulating tumour cells, tissue factor, endothelin-1 and overall survival in prostate cancer patients treated with docetaxel. Eur. J. Cancer 46, 2027–2035 (2010).
Ghossein, R. A. et al. Prognostic significance of detection of prostate-specific antigen transcripts in the peripheral blood of patients with metastatic androgen-independent prostatic carcinoma. Urology 50, 100–105 (1997).
Olsson, C. A. et al. Preoperative reverse transcriptase polymerase chain reaction for prostate specific antigen predicts treatment failure following radical prostatectomy. J. Urol. 155, 1557–1562 (1996).
Oefelein, M. G., Ignatoff, J. M., Clemens, J. Q., Watkin, W. & Kaul, K. L. Clinical and molecular followup after radical retropubic prostatectomy. J. Urol. 162, 307–310 (1999).
Thomas, J. et al. Preoperative combined nested reverse transcriptase polymerase chain reaction for prostate-specific antigen and prostate-specific membrane antigen does not correlate with pathologic stage or biochemical failure in patients with localized prostate cancer undergoing radical prostatectomy. J. Clin. Oncol. 20, 3213–3218 (2002).
Eschwege, P. et al. Prognostic value of prostate circulating cells detection in prostate cancer patients: a prospective study. Br. J. Cancer 100, 608–610 (2009).
Garcia, J. A. et al. Evaluation and significance of circulating epithelial cells in patients with hormone-refractory prostate cancer. BJU Int. 99, 519–524 (2007).
Acknowledgements
The authors thank Miriam Germann for diligently reviewing and correcting the manuscript and Till Bechtold, Oliver Borst, Sascha Hoffmann and Kai Januschowski for fruitful discussions and valuable advice while preparing the manuscript.
Author information
Authors and Affiliations
Contributions
D. Schilling and T. Todenhöfer contributed equally to researching data for the article, writing the article and reviewing the manuscript before submission. J. Hennenlotter provided a substantial contribution to researching data for the article, discussion of content and reviewing the manuscript before submission. C. Schwentner, T. Fehm and A. Stenzl provided substantial contributions to discussion of content and reviewing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Schilling, D., Todenhöfer, T., Hennenlotter, J. et al. Isolated, disseminated and circulating tumour cells in prostate cancer. Nat Rev Urol 9, 448–463 (2012). https://doi.org/10.1038/nrurol.2012.136
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrurol.2012.136
This article is cited by
-
PTEN-regulated PI3K-p110 and AKT isoform plasticity controls metastatic prostate cancer progression
Oncogene (2024)
-
CD117/c-kit defines a prostate CSC-like subpopulation driving progression and TKI resistance
Scientific Reports (2021)
-
Liquid biopsy for pediatric central nervous system tumors
npj Precision Oncology (2018)
-
MiRNA-296-3p-ICAM-1 axis promotes metastasis of prostate cancer by possible enhancing survival of natural killer cell-resistant circulating tumour cells
Cell Death & Disease (2013)