Abstract
Focal therapy is an individualized treatment option for prostate cancer, which destroys localized cancerous tissue but not normal tissue, thus avoiding the morbidities associated with whole-gland therapy. Accurate cancer localization and precise ablation are integral to the success of focal therapy, which remains unproven owing to suboptimal patient selection. Currently, there are no clinical or biopsy features that can identify unifocal prostate cancer and no imaging modality that can accurately diagnose or localize prostate cancer. MRI diagnosis has the best accuracy but high cost and limited access hinder its widespread adoption. New management options, including focal therapy and active surveillance, require prostate biopsy to detect, localize and characterize the cancer. Transrectal prostate biopsy has a high false-negative detection rate, which might be related to an inability to biopsy the anterior and apical part of the prostate or interoperator variation. Transrectal biopsy is also associated with sepsis and bleeding. Robotic transperineal prostate biopsy can overcome the limitations of transrectal procedures. Robotic biopsy is automated with high accuracy, has improved access to the apex and anterior part of the prostate and has low risk of sepsis. Furthermore, it involves only two skin punctures, compared with template-based transperineal prostate biopsy, which can result in multiple wounds. Robotic prostate biopsy fulfills the fundamental needs of focal therapy and might be the platform for future treatment delivery for prostate cancer.
Key Points
-
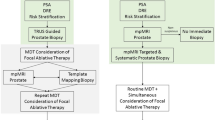
The success of focal therapy for prostate cancer is dependent on the accurate localization of cancer, in order to ensure complete ablation of known disease
-
Transrectal prostate biopsy has a high false-negative detection rate and associated risk of life-threatening infection, which can be overcome with the transperineal approach
-
As new MRI technology is developed, its accuracy for prostate cancer identification is improved
-
An image-guided robotic biopsy system will ensure accurate targeted prostate biopsy and provide a map of prostate cancer location to guide focal therapy delivery
-
Further studies are essential to verify the accuracy of targeted prostate biopsy and the clinical value of robotic and template-based transperineal approaches
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Catalona, W. J., Smith, D. S., Ratliff, T. L. & Basler, J. W. Detection of organ-confined prostate cancer is increased through prostate-specific antigen-based screening. JAMA 270, 948–954 (1993).
Cooperberg, M. R., Lubeck, D. P., Meng, M. V., Mehta, S. S. & Carroll, P. R. The changing face of low-risk prostate cancer: trends in clinical presentation and primary management. J. Clin. Oncol. 22, 2141–2149 (2004).
Caso, J. R., Mouraviev, V., Tsivian, M., Polascik, T. J. & Moul, J. W. Prostate cancer: an evolving paradigm. J. Endourol. 24, 805–809 (2010).
Andriole, G. L. et al. Mortality results from a randomized prostate cancer screening trial. N. Engl. J. Med. 360, 1310–1319 (2009).
Schröder, F. H. et al. Screening and prostate cancer mortality in a randomized European study. N. Engl. J. Med. 360, 1320–1328 (2009).
Gravas, S. & de Reijke, T. Is focal therapy an alternative to active surveillance? J. Endourol. 24, 855–860 (2010).
Hu, J. C. et al. Comparative effectiveness of minimally invasive vs open radical prostatectomy. JAMA 302, 1557–1564 (2009).
Cahlon, O., Hunt, M. & Zelefsky, M. J. Intensity-modulated radiation therapy: supportive data for prostate cancer. Semin. Radiat. Oncol. 18, 48–57 (2008).
Klotz, L. Active surveillance with selective delayed intervention: using natural history to guide treatment in good risk prostate cancer. J. Urol. 172, S48–S50 (2004).
Jayram, E. & Eggener, S. E. Patient selection for focal therapy of prostate cancer. Curr. Opin. Urol. 19, 268–273 (2009).
Hall, G. S., Kramer, C. E. & Epstein, J. I. Evaluation of radical prostatectomy specimens: a comparative analysis of sampling methods. Am. J. Surg. Pathol. 16, 315–324 (1992).
Ahmed, H. U. The index lesion and the origin of prostate cancer. N. Engl. J. Med. 361, 1704–1706 (2009).
Karavitakis, M., Ahmed, H. U., Abel, P. D., Hazell, S. & Winkler, M. H. Tumor focality in prostate cancer: implications for focal therapy. Nat. Rev. Clin. Oncol. 8, 48–55 (2011).
Mouraviev, V. et al. Prostate cancer laterality as a rationale of ablative therapy for the treatment of clinically localized prostate. Cancer 110, 906–910 (2007).
Tareen, B. et al. Appropriate candidates for hemiablative focal therapy are infrequently encountered among men selected for radical prostatectomy in contemporary cohort. Urology 73, 351–354 (2009).
Ahmed, H. U. & Emberton, M. Benchmarks for success in focal therapy of prostate cancer. World J. Urol. 28, 577–582 (2010).
D'Amico, A. V. et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy or interstitial radiation therapy for clinically localized prostate cancer. JAMA 280, 969–974 (1998).
No authors listed. Consensus statement: guidelines for PSA following radiation therapy. American Society for Therapeutic Radiology and Oncology Consensus Panel. Int. J. Radiat. Oncol. Biol. Phys. 37, 1035–1041 (1997).
Stamey, T. A. et al. Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. N. Engl. J. Med. 317, 909–916 (1987).
Ahmed, H. U., Moore, C., Lecornet, E. & Emberton, M. Focal therapy in prostate cancer: determinants of success and failure. J. Endourol. 24, 819–825 (2010).
Dall'Era, M. A. et al. Active surveillance for early-stage prostate cancer: review of the current literature. Cancer 112, 1650–1659 (2008).
Kim, C. K., Park, B. K., Lee, H. M., Kim, S. S. & Kim, E. MRI techniques for prediction of local tumor progression after high-intensity focused ultrasonic ablation of prostate cancer. AJR Am. J. Roentgenol. 190, 1180–1186 (2008).
Rouvière, O. et al. Prostate cancer transrectal HIFU ablation: detection of local recurrences using T2-weighted and dynamic contrast-enhanced MRI. Eur. Radiol. 20, 48–55 (2010).
Vellet, A. D. et al. Prostatic cryosurgery: use of MR imaging in evaluation of success and technical modifications. Radiology 203, 653–659 (1997).
Pallwein, L. et al. Value of contrast-enhanced ultrasound and elastography in imaging of prostate cancer. Curr. Opin. Urol. 17, 39–47 (2007).
Kitajima, K. et al. Prostate cancer detection with 3T MRI: comparison of diffusion-weighted imaging and dynamic contrast-enhanced MRI in combination with T2-weighted imaging. J. Magn. Reson. Imaging 31, 625–631 (2010).
Pinto, F. et al. Imaging in prostate cancer diagnosis: present role and future perspectives. Urol. Int. 86, 373–382 (2011).
Levine, M. A., Ittman, M., Melamed, J. & Lepor, H. Two consecutive sets of transrectal ultrasound guided sextant biopsies of the prostate for the detection of prostate cancer. J. Urol. 159, 471–475 (1998).
Demura, T. et al. Differences in tumor core distribution between palpable and nonpalpable prostate tumors in patients diagnosed using extensive transperineal ultrasound-guided template prostate biopsy. Cancer 103, 1826–1832 (2005).
Tiara, A. V. et al. Performance of transperineal template-guided mapping biopsy in detecting prostate cancer in the initial and repeat biopsy setting. Prostate Cancer Prostatic Dis. 13, 71–77 (2010).
Djavan, B. et al. Safety and morbidity of first and repeat transrectal ultrasound guided prostate needle biopsies: results of a prospective European prostate cancer detection study. J. Urol. 166, 856–860 (2001).
Rietbergen, J. B., Kruger, A. E., Kranse, E. & Schröder, F. H. Complications of transrectal ultrasound-guided systematic sextant biopsies of the prostate: evaluation of complication rates and risk factors within a population-based screening program. Urology 49, 875–880 (1997).
Lange, D. et al. Bacterial sepsis after prostate biopsy—a new perspective. Urology 74, 1200–1205 (2009).
Hadway, P. et al. Urosepsis and bacteraemia caused by antibiotic-resisitant organisms after transrectal ultrasonography-guided prostate biopsy. BJU Int. 104, 1556–1558 (2009).
Feliciano, J. et al. The incidence of fluoroquinolone resistant infections after prostate biopsy—are fluoroquinolone still effective prophylaxis? J. Urol. 179, 952–955 (2008).
Mohan, P., Ho, H., Yuen, J. S. P., Ng, W. S. & Cheng, W. S. A 3D computer simulation to study the efficacy of transperineal versus transrectal biopsy of the prostate. Int. J. Comput. Assist. Radiol. Surg. 1, 351–360 (2007).
Kawakami, S. et al. Transrectal ultrasound-guided transperineal 14-core systematic biopsy detects apico-anterior cancer foci of T1c prostate cancer. Int. J. Urol. 11, 613–618 (2004).
Merrick, G. S. et al. The morbidity of transperineal template-guided prostate mapping biopsy. BJU Int. 101, 1524–1529 (2008).
Shandera, K. C., Thibault, G. P. & Deshon, G. E. Jr. Variability in patient preparation for prostate biopsy among American urologists. Urology 52, 644–646 (1998).
Onik, G., Miessau, M. & Bostwick, D. G. Three-dimensional prostate mapping biopsy has a potentially significant impact on prostate cancer management. J. Clin. Oncol. 27, 4321–4326 (2009).
Emiliozzi, P. et al. The value of a single biopsy with 12 transperineal cores for detecting prostate cancer in patients with elevated prostate specific antigen. J. Urol. 166, 845–849 (2001).
Elhawary, H. et al. The case for MR-compatible robotics: a review of the state of the art. Int. J. Med. Robot. 4, 105–113 (2008).
Ho, H. S. et al. Robotic ultrasound-guided prostate intervention device: system description and results from phantom studies. Int. J. Med. Robot. 5, 51–58 (2009).
Ho, H. et al. A device for precise robotic assisted transperineal saturation prostate biopsy in men with previous negative biopsy: Preliminary study [abstract V2061]. J. Urol. 179 (Suppl.), 710 (2008).
Ho, H. et al. Robotic transperineal prostate biopsy: pilot clinical study. Urology http://dx.doi.org/10.1016/j.urology.2011.07.1389
Ho, H. et al. Robotic-assisted transperineal prostate biopsy with novel device for future prostate interventions: 3-years' clinical experience [abstract 1091]. J. Urol. 183 (Suppl.), e424–e425 (2010).
Andriole, G. L. et al. Is there a better way to biopsy the prostate? Prospects for a novel transrectal systematic biopsy approach. Urology 70 (6 Suppl.), 22–26 (2007).
Megwalu, I. I. et al. Evaluation of a novel precision template-guided biopsy system for detecting prostate cancer. BJU Int. 102, 546–550 (2008).
Natarajan, S. et al. Clinical application of a 3D ultrasound-guided prostate biopsy system. Urol. Oncol. 29, 334–342 (2011).
Gassert, R. et al. A 2-DOF fMR-compatible haptic interface to investigate the neural control of arm movements. Proceedings of the IEEE International Conference 3825–3831 (2006).
Masamune, K. et al. Development of an MRI-compatible needle insertion manipulator for stereotactic neurosurgery. J. Image Guid. Surg. 1, 242–248 (1995).
Stoianovici, D., Patriciu, A., Petrisor, D., Mazilu, D. & Kavoussi, L. A new type of motor: pneumatic step motor. IEEE/ASME Trans. Mechatronics 12, 98–106 (2007).
Fischer, G. S., DiMaio, S., Iordachita, I. I. & Fichtinger, G. Robotic assistant for transperineal prostate intervention in 3T closed MRI. Med. Image Comput. Comput. Assist. Interv. 10, 425–433 (2007).
Zangos, S. et al. MR-compatible assistance system for punction in a high-field system: device and feasibility of transgluteal biopsies of the prostate gland. Eur. Radiol. 17, 1118–1124 (2007).
Zangos, S. et al. MR-compatible assistance system for biopsy in a high-field-strength system: initial results in patients with suspicious prostate lesions. Radiology 259, 903–910 (2011).
Engelhard, K. et al. Prostate biopsy in the supine position in a standard 1.5-T scanner under real time MR-imaging control using a MR-compatible endorectal biopsy device. Eur. Radiol. 16, 1237–1243 (2006).
Singh, A. K. et al. Initial clinical experience with real-time transrectal ultrasonography-magnetic resonance imaging fusion-guided prostate biopsy. BJU Int. 101, 841–845 (2008).
Ukimura, O. et al. Technique for a hybrid system of real-time transrectal ultrasound with preoperative magnetic resonance imaging in the guidance of targeted prostate biopsy. Int. J. Urol. 10, 890–893 (2010).
Author information
Authors and Affiliations
Contributions
H. Ho, J. S. P. Yuen and C. W. S. Cheng contributed equally to the research, discussion of content, writing and reviewing of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors are patent holders/applicants with SingHealth Services Pte Ltd.
Rights and permissions
About this article
Cite this article
Ho, H., Yuen, J. & Cheng, C. Robotic prostate biopsy and its relevance to focal therapy of prostate cancer. Nat Rev Urol 8, 579–585 (2011). https://doi.org/10.1038/nrurol.2011.131
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrurol.2011.131
This article is cited by
-
Beyond transrectal ultrasound-guided prostate biopsies: available techniques and approaches
World Journal of Urology (2019)
-
Image-guided robotic interventions for prostate cancer
Nature Reviews Urology (2013)
-
Fast and robust extraction of surrogate respiratory signal from intra-operative liver ultrasound images
International Journal of Computer Assisted Radiology and Surgery (2013)