Abstract
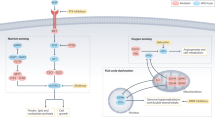
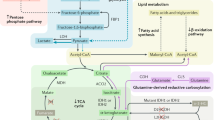
Kidney cancer is not a single disease but comprises a number of different types of cancer that occur in the kidney, each caused by a different gene with a different histology and clinical course that responds differently to therapy. Each of the seven known kidney cancer genes, VHL, MET, FLCN, TSC1, TSC2, FH and SDH, is involved in pathways that respond to metabolic stress or nutrient stimulation. The VHL protein is a component of the oxygen and iron sensing pathway that regulates hypoxia-inducible factor (HIF) levels in the cell. HGF–MET signaling affects the LKB1–AMPK energy sensing cascade. The FLCN–FNIP1–FNIP2 complex binds AMPK and, therefore, might interact with the cellular energy and nutrient sensing pathways AMPK–TSC1/2–mTOR and PI3K–Akt–mTOR. TSC1–TSC2 is downstream of AMPK and negatively regulates mTOR in response to cellular energy deficit. FH and SDH have a central role in the mitochondrial tricarboxylic acid cycle, which is coupled to energy production through oxidative phosphorylation. Mutations in each of these kidney cancer genes result in dysregulation of metabolic pathways involved in oxygen, iron, energy or nutrient sensing, suggesting that kidney cancer is a disease of cell metabolism. Targeting the fundamental metabolic abnormalities in kidney cancer provides a unique opportunity for the development of more-effective forms of therapy for this disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Linehan, W. M., Walther, M. M. & Zbar, B. The genetic basis of cancer of the kidney. J. Urol. 170, 2163–2172 (2003).
Vogelstein, B. & Kinzler, K. W. Cancer genes and the pathways they control. Nat. Med. 10, 789–799 (2004).
Thompson, C. B. Attacking cancer at its root. Cell 138, 1051–1054 (2009).
Jones, R. G. & Thompson, C. B. Tumor suppressors and cell metabolism: a recipe for cancer growth. Genes Dev. 23, 537–548 (2009).
Vander Heiden, M. G., Cantley, L. C. & Thompson, C. B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324, 1029–1033 (2009).
Seizinger, B. R. et al. Von Hippel–Lindau disease maps to the region of chromosome 3 associated with renal cell carcinoma. Nature 332, 268–269 (1988).
Lerman, M. I. et al. Isolation and regional localization of a large collection (2,000) of single copy DNA fragments on human chromosome 3 for mapping and cloning tumor suppressor genes. Hum. Genet. 86, 567–577 (1991).
Glenn, G. M. et al. Screening for von hippel-lindau disease by DNA-polymorphism analysis. JAMA 267, 1226–1231 (1992).
Latif, F. et al. Molecular and genetic characterization and physical mapping of 11 new markers detecting multiallele restriction fragment length polymorphisms on the short arm of human chromosome 3. Hum. Genet. 90, 17–22 (1992).
Maher, E. R. et al. Presymptomatic diagnosis of von Hippel–Lindau disease with flanking DNA markers. J. Med. Genet. 29, 902–905 (1992).
Crossey, P. A. et al. Genetic linkage between von Hippel–Lindau disease and three microsatellite polymorphisms refines the localisation of the VHL locus. Hum. Mol. Genet. 2, 279–282 (1993).
Latif, F. et al. Identification of the von Hippel–Lindau disease tumor suppressor gene. Science 260, 1317–1320 (1993).
Stolle, C. et al. Improved detection of germline mutations in the von Hippel–Lindau disease tumor suppressor gene. Hum. Mutat. 12, 417–423 (1998).
Gnarra, J. R. et al. Mutations of the VHL tumour suppressor gene in renal carcinoma. Nat. Genet. 7, 85–90 (1994).
Nickerson, M. L. et al. Improved identification of von Hippel–Lindau gene alterations in clear cell renal tumors. Clin. Cancer Res. 14, 4726–4734 (2008).
Shuin, T. et al. Frequent somatic mutations and loss of heterozygosity of the von Hippel-lindau tumor suppressor gene in primary human renal cell carcinomas. Cancer Res. 54, 2852–2855 (1994).
Duan, D. R. et al. Inhibition of transcription elongation by the VHL tumor suppressor protein. Science 269, 1402–1406 (1995).
Pause, A. et al. The von Hippel–Lindau tumor-suppressor gene product forms a stable complex with human CUL-2, a member of the Cdc53 family of proteins. Proc. Natl Acad. Sci. USA 94, 2156–2161 (1997).
Kibel, A., Iliopoulos, O., DeCaprio, J. A. & Kaelin, W. G. Jr. Binding of the von Hippel–Lindau tumor suppressor protein to elongin B and C. Science 269, 1444–1446 (1995).
Iliopoulos, O., Jiang, C., Levy, A. P., Kaelin, W. G. & Goldberg, M. A. Negative regulation of hypoxia-inducible genes by the von Hippel–Lindau protein. Proc. Natl Acad. Sci. USA 93, 10595–10599 (1996).
Maxwell, P. H. et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399, 271–275 (1999).
Kaelin, W. G. Jr. Molecular basis of the VHL hereditary cancer syndrome. Nat. Rev. Cancer 2, 673–682 (2002).
Kondo, K., Klco, J., Nakamura, E., Lechpammer, M. & Kaelin, W. G. Jr. Inhibition of HIF is necessary for tumor suppression by the von Hippel–Lindau protein. Cancer Cell 1, 237–246 (2002).
Kondo, K., Kim, W. Y., Lechpammer, M. & Kaelin, W. G. Jr. Inhibition of HIF2alpha is sufficient to suppress pVHL-defective tumor growth. PLoS Biol. 1, E83 (2003).
Maranchie, J. K. et al. The contribution of VHL substrate binding and HIF1-alpha to the phenotype of VHL loss in renal cell carcinoma. Cancer Cell 1, 247–255 (2002).
Gordan, J. D. et al. HIF-alpha effects on c-Myc distinguish two subtypes of sporadic VHL-deficient clear cell renal carcinoma. Cancer Cell 14, 435–446 (2008).
Thomas, G. V. et al. Hypoxia-inducible factor determines sensitivity to inhibitors of mTOR in kidney cancer. Nat. Med. 12, 122–127 (2006).
Sun, M., Lughezzani, G., Perrotte, P. & Karakiewicz, P. I. Treatment of metastatic renal cell carcinoma. Nat. Rev. Urol. (in press).
Wilhelm, S. M. et al. BAY 43–9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 64, 7099–7109 (2004).
Mendel, D. B. et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin. Cancer Res. 9, 327–337 (2003).
Rixe, O. et al. Axitinib treatment in patients with cytokine-refractory metastatic renal-cell cancer: a phase II study. Lancet Oncol. 8, 975–984 (2007).
Harris, P. A. et al. Discovery of 5-[[4-[(2, 3-dimethyl-2H-indazol-6-yl)methylamino]-2-pyrimidinyl]amino]-2-m ethyl-benzenesulfonamide (Pazopanib), a novel and potent vascular endothelial growth factor receptor inhibitor. J. Med. Chem. 51, 4632–4640 (2008).
Rapisarda, A. et al. Identification of small molecule inhibitors of hypoxia-inducible factor 1 transcriptional activation pathway. Cancer Res. 62, 4316–4324 (2002).
Rapisarda, A. et al. Topoisomerase I-mediated inhibition of hypoxia-inducible factor 1: mechanism and therapeutic implications. Cancer Res. 64, 1475–1482 (2004).
National Cancer Institute. A pilot trial of oral topotecan for the treatment of refractory advanced solid neoplasms expressing HIF-1 alpha. NCI-05-C-0186. CCR Clinical Trials at NIH [online], (2009).
Woldemichael, G. M. et al. Development of a cell-based reporter assay for screening of inhibitors of hypoxia-inducible factor 2-induced gene expression. J. Biomol. Screen. 11, 678–687 (2006).
Thomas, G. V. et al. Hypoxia-inducible factor determines sensitivity to inhibitors of mTOR in kidney cancer. Nat. Med. 12, 122–127 (2006).
Toschi, A., Lee, E., Gadir, N., Ohh, M. & Foster, D. A. Differential dependence of hypoxia-inducible factors 1 alpha and 2 alpha on mTORC1 and mTORC2. J. Biol. Chem. 283, 34495–34499 (2008).
Chresta, C. M. et al. AZD8055 is a potent, selective, and orally bioavailable ATP-competitive mammalian target of rapamycin kinase inhibitor with in vitro and in vivo antitumor activity. Cancer Res. 70, 288–298 (2010).
Sanchez, M., Galy, B., Muckenthaler, M. U. & Hentze, M. W. Iron-regulatory proteins limit hypoxia-inducible factor-2alpha expression in iron deficiency. Nat. Struct. Mol. Biol. 14, 420–426 (2007).
Rouault, T. A. The role of iron regulatory proteins in mammalian iron homeostasis and disease. Nat. Chem. Biol. 2, 406–414 (2006).
Rouault, T. A. & Tong, W. H. Iron-sulfur cluster biogenesis and human disease. Trends Genet. 24, 398–407 (2008).
Chen, G., Fillebeen, C., Wang, J. & Pantopoulos, K. Overexpression of iron regulatory protein 1 suppresses growth of tumor xenografts. Carcinogenesis 28, 785–791 (2007).
Zimmer, M. et al. Small-molecule inhibitors of HIF-2a translation link its 5'UTR iron-responsive element to oxygen sensing. Mol. Cell 32, 838–848 (2008).
Turcotte, S. et al. A molecule targeting VHL-deficient renal cell carcinoma that induces autophagy. Cancer Cell 14, 90–102 (2008).
Peruzzi, B. & Bottaro, D. P. Targeting the c-Met signaling pathway in cancer. Clin. Cancer Res. 12, 3657–3660 (2006).
Schmidt, L. S. et al. Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nat. Genet. 16, 68–73 (1997).
Schmidt, L. S. et al. Novel mutations of the MET proto-oncogene in papillary renal carcinomas. Oncogene 18, 2343–2350 (1999).
Lubensky, I. A. et al. Hereditary and sporadic papillary renal carcinomas with c-met mutations share a distinct morphological phenotype. Am. J. Pathol. 155, 517–526 (1999).
Srinivasan, R. et al. A phase II study of two dosing regimens of GSK 1363089 (GSK089), a dual MET/VEGFR2 inhibitor, in patients (pts) with papillary renal carcinoma (PRC) [abstract 5103]. J. Clin. Oncol. 27 (Suppl.), 15s (2009).
Crino, P. B., Nathanson, K. L. & Henske, E. P. The tuberous sclerosis complex. N. Engl. J. Med. 355, 1345–1356 (2006).
Bjornsson, J., Short, M. P., Kwiatkowski, D. J. & Henske, E. P. Tuberous sclerosis-associated renal cell carcinoma. Clinical, pathological, and genetic features. Am. J. Pathol. 149, 1–8 (1996).
Schmidt, L. S. et al. Birt–Hogg–Dube syndrome, a genodermatosis associated with spontaneous pneumothorax and kidney neoplasia, maps to chromosome 17p11.2. Am. J. Hum. Genet. 69, 876–882 (2001).
Nickerson, M. L. et al. Mutations in a novel gene lead to kidney tumors, lung wall defects, and benign tumors of the hair follicle in patients with the Birt–Hogg–Dube syndrome. Cancer Cell 2, 157–164 (2002).
Birt, A. R., Hogg, G. R. & Dube, W. J. Hereditary multiple fibrofolliculomas with trichodiscomas and acrochordons. Arch. Dermatol. 113, 1674–1677 (1977).
Pavlovich, C. P. et al. Renal tumors in the Birt–Hogg–Dubé syndrome. Am. J. Surg. Pathol. 26, 1542–1552 (2002).
Pavlovich, C. P. et al. Evaluation and management of renal tumors in the Birt–Hogg–Dube syndrome. J. Urol. 173, 1482–1486 (2005).
Toro, J. R. et al. Lung cysts, spontaneous pneumothrorax and genetic associations in 89 families with Birt–Hogg–Dubé syndrome. Am. J. Respir. Crit. Care Med. 175, 1044–1053 (2007).
Schmidt, L. S. et al. Germline BHD-mutation spectrum and phenotype analysis of a large cohort of families with Birt–Hogg–Dubé syndrome. Am. J. Hum. Genet. 76, 1023–1033 (2005).
Toro, J. R. et al. BHD mutations, clinical and molecular genetic investigations of Birt–Hogg–Dube syndrome: a new series of 50 families and a review of published reports. J. Med. Genet. 45, 321–331 (2008).
Vocke, C. D. et al. High frequency of somatic frameshift BHD gene mutations in Birt–Hogg–Dube-associated renal tumors. J. Natl Cancer Inst. 97, 931–935 (2005).
Baba, M. et al. Folliculin encoded by the BHD gene interacts with a binding protein, FNIP1, and AMPK, and is involved in AMPK and mTOR signaling. Proc. Natl Acad. Sci. USA 103, 15552–15557 (2006).
Hasumi, H. et al. Identification and characterization of a novel folliculin-interacting protein FNIP2. Gene 415, 60–67 (2008).
Takagi, Y. et al. Interaction of folliculin (Birt–Hogg–Dube gene product) with a novel Fnip1-like (FnipL/Fnip2) protein. Oncogene 27, 5339–5347 (2008).
Hasumi, Y. et al. Homozygous loss of BHD causes early embryonic lethality and kidney tumor development with activation of mTORC1 and mTORC2. Proc. Natl Acad. Sci. USA 106, 18722–18727 (2009).
Baba, M. et al. Kidney-targeted Birt–Hogg–Dubé gene inactivation in a mouse model: Erk1/2 and Akt-mTOR activation, cell hyperproliferation, and polycystic kidneys. J. Natl Cancer Inst. 100, 140–154 (2008).
Chen, J. et al. Deficiency of FLCN in mouse kidney led to development of polycystic kidneys and renal neoplasia. PLoS ONE 3, e3581 (2008).
Tomlinson, I. P. et al. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat. Genet. 30, 406–410 (2002).
Merino, M. J., Torres-Cabala, C., Pinto, P. A. & Linehan, W. M. The morphologic spectrum of kidney tumors in hereditary leiomyomatosis and renal cell carcinoma (HLRCC) syndrome. Am. J. Surg. Pathol. 31, 1578–1585 (2007).
Stewart, L. et al. Association of germline mutations in the fumarate hydratase gene and uterine fibroids in women with hereditary leiomyomatosis and renal cell cancer. Arch. Dermatol. 144, 1584–1592 (2008).
Toro, J. R. et al. Mutations in the fumarate hydratase gene cause hereditary leiomyomatosis and renal cell cancer in families in North America. Am. J. Hum. Genet. 73, 95–106 (2003).
Wei, M. H. et al. Novel mutations in FH and expansion of the spectrum of phenotypes expressed in families with hereditary leiomyomatosis and renal cell cancer. J. Med. Genet. 43, 18–27 (2006).
Isaacs, J. S. et al. HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: novel role of fumarate in regulation of HIF stability. Cancer Cell 8, 143–153 (2005).
Yang, Y. et al. UOK 262 cell line, fumarate hydratase deficient (FH-/FH-) hereditary leiomyomatosis renal cell carcinoma: in vitro and in vivo model of an aberrant energy metabolic pathway in human cancer. Cancer Genet. Cytogenet. 196, 45–55 (2010).
Sudarshan, S. et al. Fumarate hydratase deficiency in renal cancer induces glycolytic addiction and HIF-1{alpha} stabilization by glucose-dependent generation of reactive oxygen species. Mol. Cell Biol. 15, 4080–4090 (2009).
Xie, H. et al. LDH-A inhibition, a therapeutic strategy for treatment of hereditary leiomyomatosis and renal cell cancer. Mol. Cancer Ther. 8, 626–635 (2009).
Baysal, B. E. et al. Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science 287, 848–851 (2000).
Vanharanta, S. et al. Early-onset renal cell carcinoma as a novel extraparaganglial component of SDHB-associated heritable paraganglioma. Am. J. Hum. Genet. 74, 153–159 (2004).
Neumann, H. P. et al. Distinct clinical features of paraganglioma syndromes associated with SDHB and SDHD gene mutations. JAMA 292, 943–951 (2004).
Ricketts, C. et al. Germline SDHB mutations and familial renal cell carcinoma. J. Natl Cancer Inst. 100, 1260–1262 (2008).
Srirangalingam, U. et al. Clinical manifestations of familial paraganglioma and phaeochromocytomas in succinate dehydrogenase B (SDH-B) gene mutation carriers. Clin. Endocrinol. (Oxf.) 69, 587–596 (2008).
Henderson, A., Douglas, F., Perros, P., Morgan, C. & Maher, E. R. SDHB-associated renal oncocytoma suggests a broadening of the renal phenotype in hereditary paragangliomatosis. Fam. Cancer 8, 257–260 (2009).
Motzer, R. J. et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J. Clin. Oncol. 27, 3584–3590 (2009).
Warburg, O. On the origin of cancer cells. Science 123, 309–314 (1956).
Krishnan, B. & Truong, L. D. Renal epithelial neoplasms: the diagnostic implications of electron microscopic study in 55 cases. Hum. Pathol. 33, 68–79 (2002).
Landman, G. W. et al. Metformin associated with lower cancer mortality in type 2 diabetes: ZODIAC-16. Diabetes Care 33, 322–326 (2010).
Evans, J. M., Donnelly, L. A., Emslie-Smith, A. M., Alessi, D. R. & Morris, A. D. Metformin and reduced risk of cancer in diabetic patients. BMJ 330, 1304–1305 (2005).
Hirsch, H. A., Iliopoulos, D., Tsichlis, P. N. & Struhl, K. Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Res. 69, 7507–7511 (2009).
Huang, X. et al. Important role of the LKB1-AMPK pathway in suppressing tumorigenesis in PTEN-deficient mice. Biochem. J. 412, 211–221 (2008).
Chow, W. H., Gridley, G., Fraumeni, F. J. & Jarvholm, B. Obesity, hypertension, and the risk of kidney cancer in men. N. Engl. J. Med. 343, 1305–1311 (2000).
Leitao, V. A. et al. Renal medullary carcinoma. Case report and review of the literature. Urol. Int. 77, 184–186 (2006).
Davis, C. J. Jr, Mostofi, F. K. & Sesterhenn, I. A. Renal medullary carcinoma. The seventh sickle cell nephropathy. Am. J. Surg. Pathol. 19, 1–11 (1995).
Linehan, W. M. et al. Molecular diagnosis and therapy of kidney cancer. Annu. Rev. Med. 10, 329–343 (2010).
Jaakkola, P. et al. Targeting of HIF-alpha to the von Hippel–Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292, 468–472 (2001).
Acknowledgements
This research was supported in part by the Intramural Research Program of the National Institute of Health, National Cancer Institute, Center for Cancer Research and funded in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Heath and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. The authors acknowledge the outstanding editorial and graphics support by Georgia Shaw.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Linehan, W., Srinivasan, R. & Schmidt, L. The genetic basis of kidney cancer: a metabolic disease. Nat Rev Urol 7, 277–285 (2010). https://doi.org/10.1038/nrurol.2010.47
Issue Date:
DOI: https://doi.org/10.1038/nrurol.2010.47
This article is cited by
-
Metabolic alterations in hereditary and sporadic renal cell carcinoma
Nature Reviews Nephrology (2024)
-
The multi-omics analyses of acsl1 reveal its translational significance as a tumor microenvironmental and prognostic biomarker in clear cell renal cell carcinoma
Diagnostic Pathology (2023)
-
Identification of BRAF, CCND1, and MYC mutations in a patient with multiple primary malignant tumors: a case report and review of the literature
World Journal of Surgical Oncology (2023)
-
Glycolysis-related biomarker TCIRG1 participates in regulation of renal cell carcinoma progression and tumor immune microenvironment by affecting aerobic glycolysis and AKT/mTOR signaling pathway
Cancer Cell International (2023)
-
Candidate variants in DNA replication and repair genes in early-onset renal cell carcinoma patients referred for germline testing
BMC Genomics (2023)